Why the most ‘potent’ homeopathic dilutions of arnica
don’t actually contain any arnica
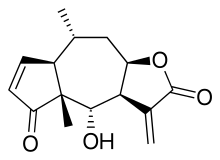
One of the chemical compounds in arnica which has been
associated with pharmacological activity is helenalin:
In chemistry there is a specific amount of substance
known as the mole. The mole is
significant because (in the case of molecular compounds) it is that particular
amount of the compound which has an actual mass in grams numerically equal to
the relative molecular mass. For helenalin this is
261.31 grams (just over a quarter of a kilogram).
One mole of any simple molecular compound always contains the same number of molecules, approximately 602,200,000,000,000,000,000,000 molecules, which is abbreviated as 6.022 x 1023 molecules. A lot of molecules!
(Note
that if we’d rounded the number of molecules down to
600,000,000,000,000,000,000,000, the 23 in 1023 would have
represented the number of noughts.)
The concentration of a solution is often measured in
moles per dm3 i.e. mol dm-3. In
everyday language a dm3 is more often known as a litre (a large
glass of European draft beer) and a solution containing 1 mole of a substance in 1
litre of solution is often set as a standard in chemistry. This is known as a 1
molar solution; that is, it contains 1 mol dm-3
or 1 mole per litre of solution.
It would be impossible for a starting solution of arnica
to contain 1 mole per litre of helenalin (over ¼ kg
in 1 litre of solution), but let’s say it did.
That would mean that the solution contained 6.022 x 1023
molecules per litre.
Each homeopathic (C) dilution by 100 reduces the number of
molecules per litre by a factor of 100. That is, it knocks off 2 of the
noughts in 602,200,000,000,000,000,000,000 each time, or, to consider it the other way,
it reduces the power to which 10 is raised by 2 each time - the first dilution would
reduce the number of molecules per litre from 6.022 x 1023 to 6.022 x 1021.
By the time you get to 12 homeopathic dilutions (dilution by a factor of 1024), you are down to 0.6 (i.e. less than 1 molecule) in 1 litre of solution. By the time you get to the (commonly regarded as potent) 30C homeopathic dilution (dilution by a factor of 1060), you’d be lucky to find 1 molecule in 1036 litres of solution (1/1024-60).
For comparison, the volume of
all the earth’s oceans is only about 1.4 x 1021 litres, the volume
of the whole earth is only about 1.098 x 1024 litres
, the volume of our galaxy (the Milky Way) is estimated at 6.65 x 1063
litres and the volume of the entire, so-called, observable universe is estimated by one source at about 3.5 x 1083
litres.
(Remember that each time you add 1 to the power to which 10 is raised, you end up multiplying some
already unimaginable numbers by 10!!!)
All this is great news for the capitalist organisations that sell homeopathic remedies, because they hardly need any of the stuff that customers imagine they’re getting.
Of course, this is but theory. What's more important is the empirical evidence which largely confirms that placebos are generally at least as effective homeopathic preparations.
(Please send any comments, queries, or corrections to info@skilldoctors.co.uk )
Chemistry In Perspective: Full Contents
© 2017 Adrian Faiers MA (Oxon) MCIPR