Recommended by:

Top 20 UK science resources,
The Tutor Website
Recommended by:

Rated:

2010
Listed on the science, engineering and technology section of

'providing you with access to the very best Web resources for education and research, evaluated and selected by a network of subject specialists.'
(Please note that intute closed in July 2011)
Section 1: Essentials (physical chemistry)
CHAPTER 11: REDOX
EQUILIBRIA
AND ELECTROCHEMISTRY
NB This chapter has now been
updated to improve browser compatibility.
Please
use the 'send email' link at the top right hand corner of this page to
report any problems.
11.1. THE HORROR
The mere mention of redox potentials is enough to send even competent students into a state of panic. The reason is laudible. By the time they encounter this area of chemistry, students expect the theory to be totally logical. For whatever reason, nobody will tell them: electrochemistry is governed by a set of totally arbitrary conventions.
The conventions are so arbitrary, it could be argued that if the coin had landed the right way up, we might have a set of conventions completely opposite to those which have been adopted internationally (by IUPAC, the International Union of Pure and Applied Chemistry).
While this is sinking in, and before we look at it in more detail, we shall limber up by clarifying some relatively logical background theory. Moreover, when we do examine the arbitrary conventions, we shall limit ourselves to three, and systematically develop the remaining theory from these three (IUPAC consistent) conventions.
11.2. OXIDATION AND REDUCTION
11.2.1. Not surprisingly, redox potentials (or electrode potentials) are concerned with oxidation and reduction reactions. Like positive and negative, or left and right, oxidation and reduction will ferret out any part of our brain which we have decided to reserve for dyslexia. Since electrochemistry involves all three troublesome pairs (left/right, positive/negative, and oxidation/reduction), it is essential to have some solid reminders of what's what.
11.2.2. Reduction,
then, involves a reduction in oxidation number (or
"oxidation state").
Remember this, and we shall go on to clarify some terms.
11.3. CLARIFICATIONS
11.3.1. Oxidation is the loss of electrons by atoms, ions or groups.
It results in an increase in oxidation number/state.
11.3.2. Reduction is the gain of electrons by atoms, ions, or groups.
It results in a reduction in oxidation number/state.
11.3.3. A redox reaction is a reaction involving oxidation and reduction. The number of electrons lost in the oxidation process will obviously be equal to the number of electrons gained in the reduction process.
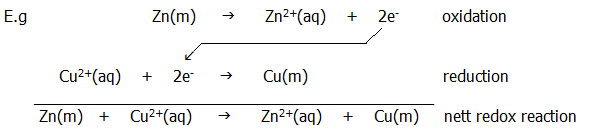
11.3.4. A disproportionation reaction is a reaction in which one substance undergoes simultaneous oxidation and reduction, some (or part) being oxidised at the expense of some (or part) being reduced (table 22.2. note ii.b.).
11.3.5. Oxidation state/oxidation number is a number which measures the state of oxidation of an atom, ion, or group.
More precisely, it is the number of electrons which the species has:
either lost (or lost control of), making it more positive, and giving it a positive oxidation state/number,
or gained (or gained control of), making it more negative and giving it a negative oxidation state/number.
Species which have neither lost/lost control of, nor gained/gained control of electrons, have an oxidation state of zero.
11.4. EXAMPLES OF OXIDATION STATE
11.4.1. Magnesium chloride, MgCl2, is ionic.
The Mg2+ ion has completely lost electrons and therefore has a positive oxidation state as well as the full blown positive charge. It has lost two electrons so the oxidation state has a numerical value of 2, as does the charge. Oxidation state is expressed in roman numerals so the magnesium ion has an oxidation state of +II, and a 2+ charge.
By comparison, each Cl- ion has completely gained one electron giving it an oxidation state of -I, and a 1- charge.
11.4.2. Water is a covalent molecule, so none of the covalently bound atoms has completely lost or gained electrons.
However, each hydrogen atom is more electropositive than the oxygen atom to which it is bound. Each has therefore lost control of its bonding electron to the oxygen atom, via the bonding orbital. This loss of control of one electron gives it an oxidation state of +I (as well as a partial positive charge).
Moreover, each oxygen atom has gained control of two electrons, one from each hydrogen atom, giving it a partial negative charge and an oxidation state of -II.
11.4.3. The tetrathionate ion, S4O62-, is particularly interesting. There is a short cut for working out oxidation states which does not work in this case.
However, the short cut is often useful and depends on a few simple rules. First, the overall oxidation state of a species is equal to its charge. For neutral atoms, molecules, or compounds, this is obviously zero. Second, certain elements nearly always have the same oxidation state when they appear in compounds. For example, oxygen usually has an oxidation state of -II, hydrogen usually has an oxidation state of +I, group I metals an oxidation state of +I, and so on.
Applying the rules to the tetrathionate ion gives a strange result:
There are 6 oxygen atoms in the ion which are therefore assumed to have a total oxidation state of 6 x -II = -XII. The overall charge of the ion is 2-, so the total oxidation state must be -II.
This means the total oxidation state of the sulphur atoms must be +X:
(+X) + (-XII) = -II, numerically equal to the charge.
However, since there are 4 sulphur atoms in the ion, this would mean that each sulphur atom has an apparent oxidation state of +X ¸ 4 = +IIŻ. Happily, the more rigorous method for deducing oxidation state solves the problem.
(Note the practical advantage of using roman numerals for oxidation states. It avoids confusion between oxidation states, and numbers of atoms/moles/etc.)
11.4.4. Another
go at tetrathionate.
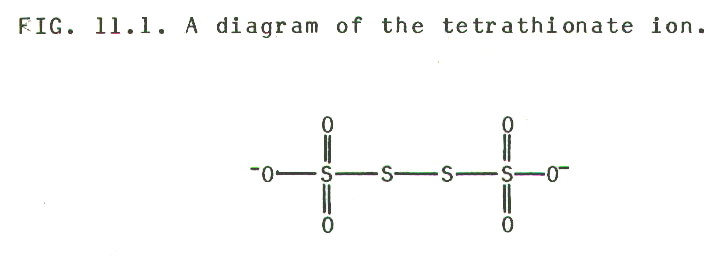
Oxygen is more electronegative
than sulphur, as you would predict from its relative position in the
periodic table. Each of the two sulphur atoms shown one at either end
of the ion (FIG. 11.1.) has five covalent bonds with oxygen atoms. It
therefore follows that each has lost control of five electrons to the
more electronegative oxygen atoms, and each has an oxidation state of
+V.
However, each of the two sulphur atoms shown in the middle of the ion (FIG. 11.1.) has two covalent bonds with other sulphur atoms. Since there is obviously no difference in electronegativity between one sulphur atom and another, the two sulphur atoms in the middle of the ion have neither lost nor gained control of bonding electrons. They are equally shared. Thus each of the central sulphur atoms has an oxidation state of zero.
The odd oxidation state of +IIŻ is therefore the average oxidation state of the four sulphur atoms in the tetrathionate ion.
11.4.5. To complete the picture, each of the oxygen atoms shown as doubly bonded to sulphur atoms (FIG. 11.1.) has gained control of two electrons via two covalent bonds with a more electropositive element, sulphur. Each therefore has an oxidation state of -II.
Each of the oxygen atoms shown carrying a negative charge (FIG. 11.1.) has gained control of one electron via a covalent bond with sulphur. Also, each has complete control of one of the electrons responsible for the negative charge (in sodium tetrathionate, for example, this has effectively come from one of the sodium ions). Each of these oxygen atoms has therefore gained control of one electron, and completely gained another, and thus each has an oxidation state of -II.
As it happens, the negative charge is actually delocalised, and there are no doubly bonded or singly bonded oxygen atoms in the ion. However, this merely demonstrates that a model which is inaccurate in one respect may be useful in another.
11.5. BALANCING REDOX REACTIONS
11.5.1. Simple, logical steps make the balancing of redox reactions easy, once the principles in sections 11.3. and 11.4. have been understood.
For example, *dichromate ions oxidise *sulphite ions to *sulphate ions in acid conditions (*using old names so as not to give too much away at this stage). The dichromate ions are themselves reduced to Cr3+ ions. The following procedure makes balancing this, and other redox reactions, simple.
STEP 1: Write an
equation showing the oxidised and reduced species, plus oxidation
states of the relevant atoms and ions. Even when it gives fractional
oxidation states, the short cut method for deducing oxidation state can
be used in most cases:
FIG
11.2 (Click on diagram to enlarge on a new web page)
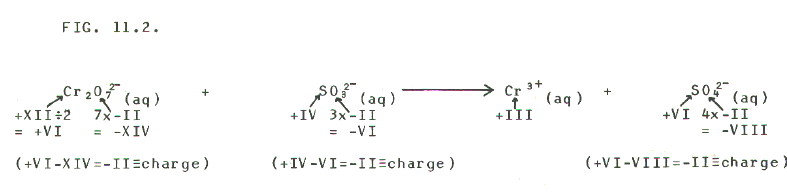
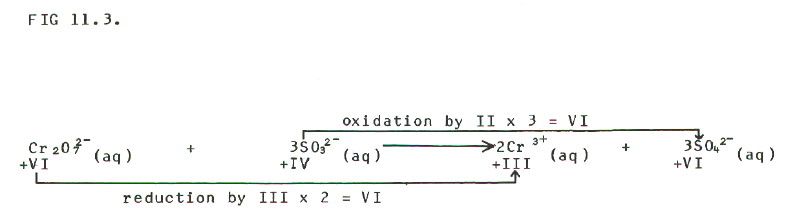
STEP 2: Balance oxidation with
reduction: the increase in oxidation state (electrons lost in the
oxidation process) must obviously equal the decrease in oxidation state
(electrons gained in the reduction process):
FIG
11-3 (Click on diagram to enlarge on a new web page)
STEP 3: Balance oxygens with
water:
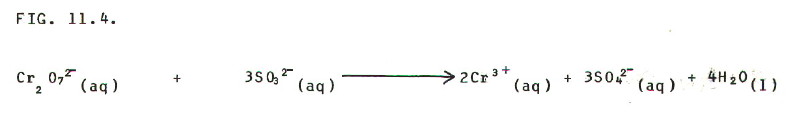
STEP 4: Balance hydrogens with
hydrogen ions:
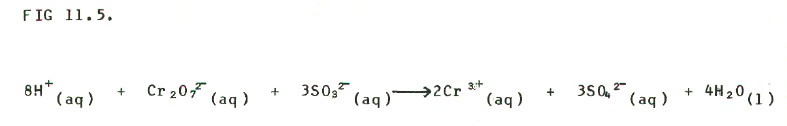
FINAL CHECK: You will see that
by this stage the charge on both sides of the equation balances.
It can also be seen why dichromate ions are more effective at oxidising in acid conditions. Do you think the high hydrogen ion concentration affects rate, or equilibrium, or both?
11.5.2. In alkaline conditions the above method needs modification. For example, manganate(VII) ions oxidise sulphate(IV) ions to sulphate(VI) ions in alkaline conditions. The manganate(VII) ions are themselves reduced to manganese(IV) oxide. (In acid conditions they are reduced to manganese 2+ ions.)
The simplest method for
balancing the equation is to initially follow the 4 steps shown above
for acid or neutral conditions. This gives the following equation for
the manganate/sulphate example:
FIG
11-6 (Click on diagram to enlarge on a new web page)
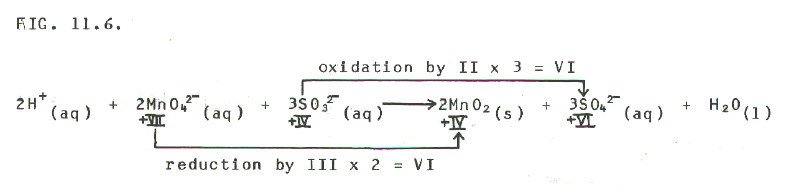
THEN STEP 5: "Remove" the
hydrogen ions by adding hydroxide ions to both sides:
FIG
11-7 (Click on diagram to enlarge on a new web page)
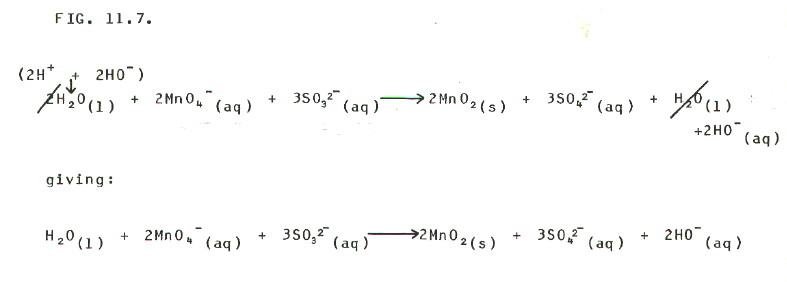
FINAL CHECK: Again, the charge
on the two sides of the equation balances by this stage.
11.6. GENTLE PREPARATION
It is time to prepare
for an attack on electrochemical cells. When a piece of metal (A) is
placed in a solution containing ions of a less electropositive metal
(B), the following reaction tends to occur:
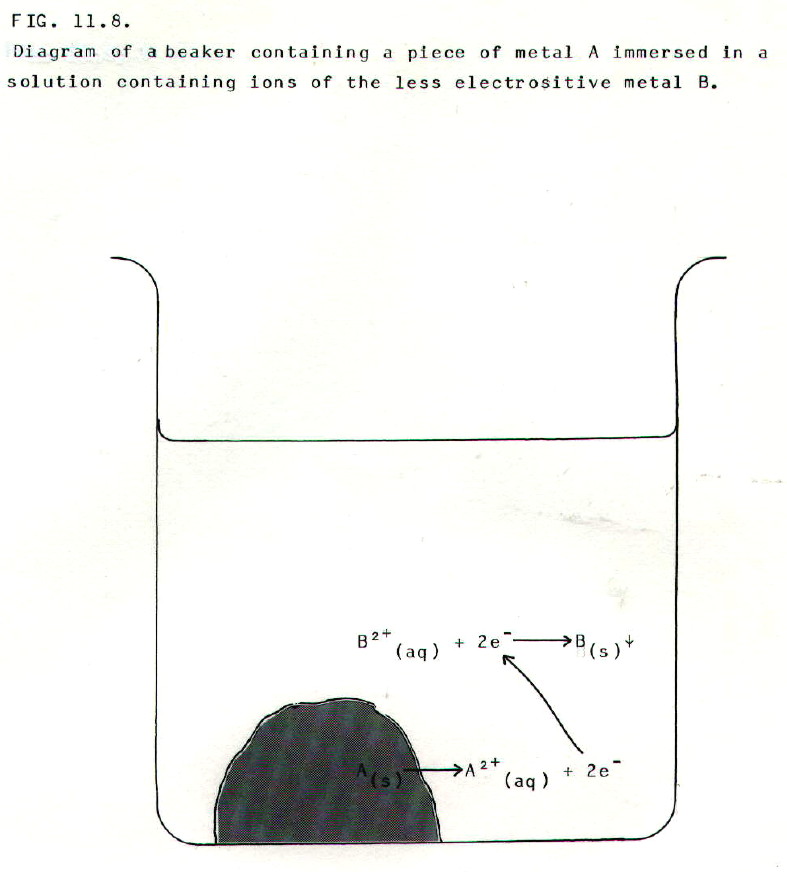
The more electropositive metal
tends to lose electrons and form positive ions (oxidation) at the
expense of the ions of the less electropositive metal. These tend to
gain electrons and form the metal (reduction).
A number of such experiments enables metals to be placed in order of electropositivity/electronegativity. The more electropositve metals (less electronegative) are also the more reactive metals. The resultant order is known as the electrochemical series, or reactivity series.
However, the experiments give no quantitative measure of relative reactivities. If it were possible to separate the two halves of the reaction (oxidation and reduction) and measure the readiness with which the electrons were transferred from one metal to another's ions, a quantitative measure could be obtained. In electrochemical cells, it is exactly this which can be done.
11.7. A SIMPLE CELL
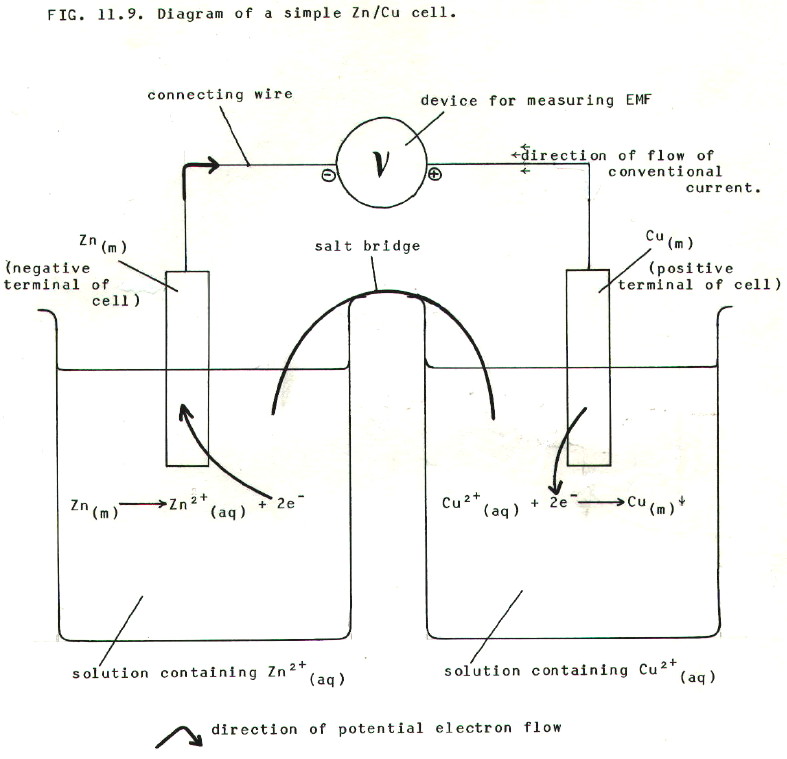
11.7.1. The above
diagram shows a simple cell which can give a quantitative
measure of the difference in reactivity between zinc and copper.
11.7.2. The salt bridge is needed to complete the circuit by allowing passage of ions (and therefore transport of charge) between the two beakers of solution. A salt bridge can be as simple as a piece of filter paper soaked in ionic solution, but the ionic solution chosen must not react with the solutions in either beaker, nor must it contain any of the ions involved in the cell reaction.
Another type of salt bridge has the connecting ionic solution as a gel in a glass U-tube. Alternatively, ionic contact between the two halves of the cell can be established in a more subtle way, with the solution for one half of the cell held in a porous pot, which is immersed in a container holding the solution for the other half of the cell. Gels and pastes make even the porous pot unnecessary. Try and think of some more variations.
11.7.3. The device for measuring EMF is the key to obtaining a quantitative measure of relative reactivities. EMF is the electrical potential difference when no current is flowing. If current were to flow, energy would be dissipated in overcoming electrical resistance in the circuit. In contrast, EMF measures the maximum energy with which electrons can be transferred in the cell between zinc metal and copper ions.
The actual device used can be a potentiometer set up, or a high restistance voltmeter, such as a valve voltmeter. Hopefully, you will have encountered potentiometers in A-level physics. Their essential property is that they balance the EMF of another cell against the EMF of the cell to be measured, thus preventing the flow of current. The essential property of a high resistance voltmeter is its extremely high sensitivity. It therefore requires negligible current. The high resistance is a secondary property which ensures the minimal flow of current.
11.8. STANDARDS
11.8.1. For comparison of one cell's EMF with another's, the conditions under which measurements are made must be standardised. The standards adopted are similar to those for enthalpy measurements and so on:
i) a temperature of 298K;
ii) atmospheric pressure;
iii) solutions one molar with respect to participating ions;
iv) solids must be pure.
The standard EMF
measured under such conditions is represented as 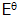 , with the familiar
"bus stop" sign. Under such conditions, the EMF of the Zn/Cu cell is
1.10V.
, with the familiar
"bus stop" sign. Under such conditions, the EMF of the Zn/Cu cell is
1.10V.
11.9. CELL DIAGRAMS
Chemical terminology
reaches a pinnacle of confusion here, and just as we are about to
encounter the first arbitrary convention. The fact is that cell
diagrams are not the same as diagrams of cells. A diagram of
a cell (Zn/Cu) is shown in FIG. 11.9. A cell diagram
for the same Zn/Cu cell is shown below in FIG. 11.10.:
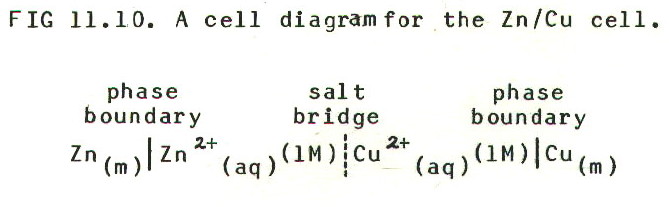
11.10 THE FIRST ARBITRARY CONVENTION
In writing the cell diagram we have employed the first of the three arbitrary conventions we mentioned in section 11.1.
11.10.1. The first arbitrary convention is this: when writing cell diagrams, the more electropositive electrode (somewhat confusingly, the one with the more *negative electrode potential) is placed on the left hand side.
*We shall see why electropositive electrodes have negative electrode potentials in section 11.12. As implied in section 11.1, arguably better arbitrary decisions could have been made giving positive electrode potentials for electropositve electrodes. Never mind, there is still a considerable amount of logic in the cell diagram and, moreover, there are several points which follow logically from it:
FIRST: Considering the
equation for the cell reaction, the species in each half of the cell
are written in a logical order:
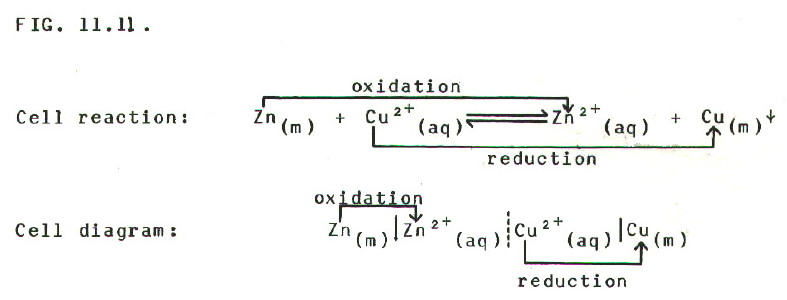
SECOND: The reaction which will
*tend to occur on the LHS is oxidation. This produces
electrons, making the LH electrode the negative terminal
of the cell.
THIRD: The reaction which will *tend to occur on the RHS is reduction. This uses electrons, making the RH electrode the positive terminal of the cell.
(*We say tend to occur, because EMF is measured with no current flowing and therefore with no nett reaction occurring. The cell is in a somewhat artificial state of equilibrium.)
FOURTH: A positive EMF should be recorded when a voltmeter is correctly connected to the cell i.e. positive terminal of the voltmeter connected to positive terminal of the cell, and negative to negative.
FIFTH: Recording a negative EMF shows us that something is the wrong way round: either the theory (we have made a mistake about which electrode is more electropositive), or the practice (e.g. we have accidentally connected the terminals of the voltmeter the wrong way round).
11.11. ANOTHER STANDARD
At this stage we have a standard method for measuring relative reactivities: a series of different cells will give a quantitative measure of the relative reactivities of a series of metals. However, we have no absolute measure of reactivity.
What we need is a
standard electrode with which to compare any other electrode. The
electrode chosen as the standard is the hydrogen electrode.
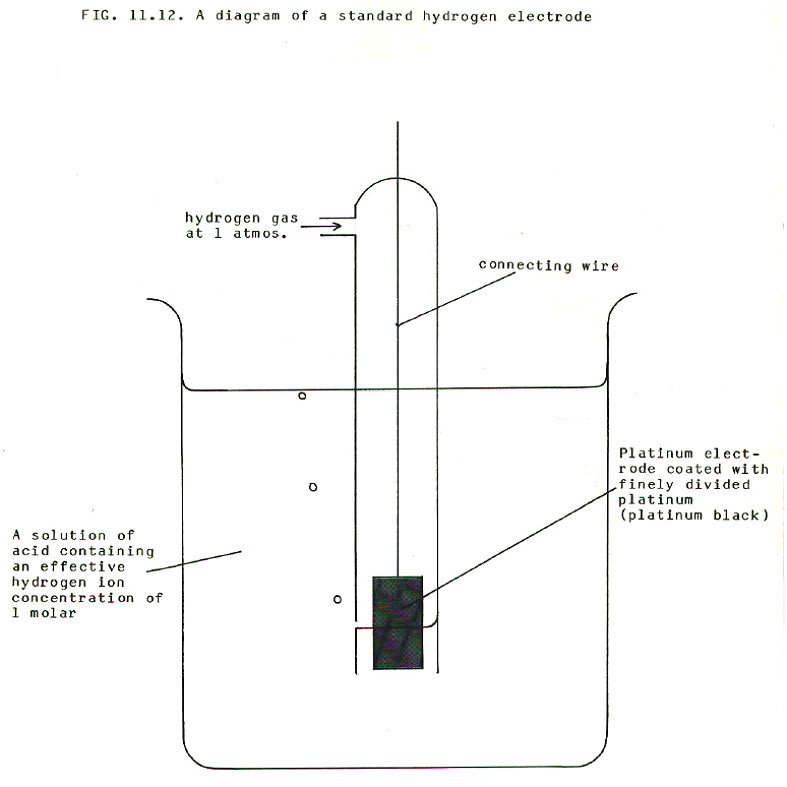
This diagram shows how the
electrode potential of a gas/ion pair can be measured. In this case the
gas is adsorbed on the platinum black surface of a platinum electrode
immersed in the appropriate solution. It is thus able to make
electrical contact with the aqueous ions and with the more conventional
electrical circuitry.
Strictly speaking the diagram shows a half-cell i.e. the electrode plus the standard solution of ions in which it is immersed. The term electrode is often used loosely to mean half-cell.
11.12. THE OTHER TWO ARBITRARY CONVENTIONS
We shall now clarify the meaning of standard electrode potential. In so doing, we shall adopt the two remaining arbitrary conventions which we chose to specify in this chapter.
The standard electrode potential for a half-cell is the EMF of a cell made up from: a standard half-cell containing the relevant electrode and ions, plus a standard hydrogen "electrode" as the left hand half-cell. It is an arbitrary measure of the potential difference between the electrode and the aqueous ions in which it is immersed e.g. between a metal and a 1 molar solution of one of its cations.
11.12.1. So, the second arbitrary convention is this: when writing cell diagrams, the hydrogen electrode is always placed on the left hand side, whether or not it is more electropositive than the other electrode.
11.12.2. The third arbitrary convention is this: the standard electrode potential for the hydrogen electrode is zero.
11.12.3. All the otherwise inexplicable facts that make electrochemistry so confusing for students at this level, now fall into place:
FIRST: Standard electrode potential measures the likelihood of reduction occurring at the electrode for which it is quoted, i.e. the right hand electrode of a cell with a standard hydrogen electrode on the left.
SECOND: A positive standard electrode potential shows that the cell is behaving as set out in section 11.10.1. i.e. the reaction tending to occur at the left hand electrode is oxidation and the reaction tending to occur at the right hand electrode is reduction. Thus the right hand half-cell contains an oxidising agent - one which is more electronegative than aqueous hydrogen ions in contact with hydrogen gas under standard conditions.
THIRD: A negative standard electrode potential shows that the cell is behaving in exactly the opposite way to that set out in section 11.10.1. i.e. the reaction tending to occur at the left hand electrode is reduction and the reaction tending to occur at the right hand electrode is oxidation. Thus the right hand half-cell contains a reducing agent - one which is more electropositive than hydrogen gas in contact with aqueous hydrogen ions under standard conditions.
FOURTH: The EMF of a cell will be the sum of the redox potentials for the two electrodes from wich it is made. Since redox potentials are quoted for reduction reactions, and since oxidation tends to occur at the left hand electrode, the sign of the redox potential for the left hand electrode potential should be reversed.
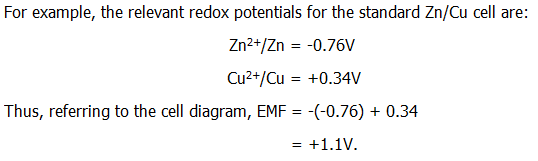
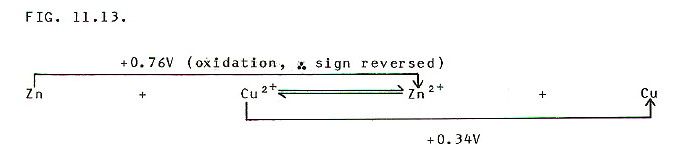
Rather than writing
cell diagrams each time you make such a calculation, it is often much
simpler to set them out like this:

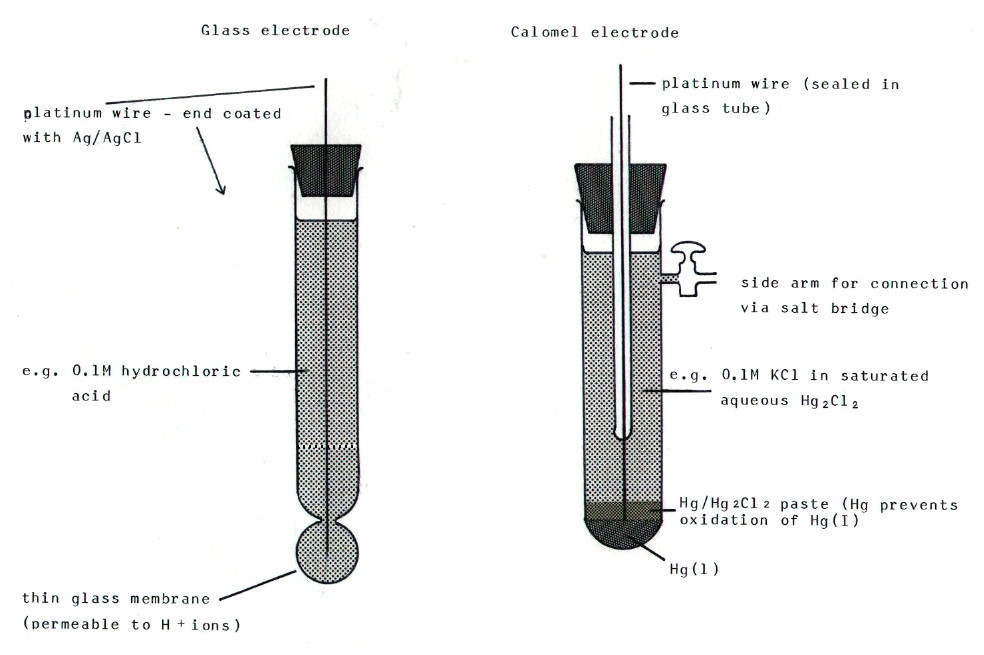
11.12.4. A quick diversion: A simple pH meter could be based on the hydrogen electrode, since its electode potential depends on the hydrogen ion concentration. In practice, the hydrogen electrode is clumsy to use and a glass electrode is used. This measures the potential difference across a glass membrane between a 0.1M solution of hydrochloric acid in the electrode and the solution of unknown pH into which it is immersed. The electrode is calibrated by immersing it in a range of buffers.
In addition, the
clumsiness of the hydrogen electrode means that it is rarely used even
as a standard, and the calomel electrode is used instead. It contains
mercury in contact with a solution of potassium chloride saturated with
mercury(I) chloride. Its standard electrode potential for various
concentrations of KCl is known over a range of temperatures, so it is
possible to calculate the standard electrode potential of the other
electrode with respect to a hydrogen electrode. Both the glass
electrode and a calomel electrode are illustrated in FIG. 11.14.
FIG
11.14 (Click on diagram to enlarge on a new web page)
11.13. FEASIBILITY OF
REACTIONS
One of the great merits of redox potentials is that they can be used to predict the feasibility of reactions. Using calculations such as the one at the end of section 11.12.3. it is possible to predict whether or not a reaction is feasible under standard conditions. Reactions which are calculated to have positive EMF values are feasible under standard conditions, those which have negative EMF values are not. Those which have an EMF of zero are at equilibrium under standard conditions.
A list of redox potentials for different electrodes (or, more correctly, half- cells) is therefore a very powerful tool. When it comes to predicting feasibility, the EMF of a cell is equalled only by the Gibbs Free Energy change for the reaction. As we shall see in the next chapter, this is more than a coincidence.
11.14. ELECTROLYSIS
11.14.1. In many ways, electrolysis can be regarded as the reverse of the process which occurs in an electrochemical cell. In this case, an electric current passed through an electrolyte causes a chemical reaction to occur. Examples of industrial electrolysis are given in sections 19.1.3., 19.1.4., and 19.3.1..
The anode is the positive electrode. It attracts negative ions (anions) and discharges them (oxidation).
The cathode is the negative electrode. It attracts positive ions (cations) and discharges them (reduction).
11.4.2. When more than one type of anion or cation is present, there are several factors that decide which ion will be preferentially discharged:
i) The position of the ion in the electrochemical series: E.g. The anion of an electronegative element is less likely to be discharged at the anode than is the anion of a less electronegative element. The cation of an electropositive element is less likely to be discharged at the cathode than is the cation of a less electropositive element.
ii) Concentration: The more concentrated an ion in the electrolyte, the more likely it is to be discharged. This may override relative positions in the electrochemical series (section 19.3.1.).
iii) The nature of the electrodes: For example, the standard hydrogen electrode measures the potential for discharge of hydrogen at a platinum surface. However, platinum catalyses the formation of hydrogen gas molecules from the hydrogen atoms produced by discharge of hydrogen ions. Mercury does not catalyse this step, and hydrogen is said to have an overpotential at a mercury surface (section 19.3.1.).
Moreover, when a copper anode is used in the electrolysis of sulphuric acid, copper metal from the anode is oxidised to copper ions in preference to the discharge of hydrogen ions (section 19.1.9.).
11.14.3. Calculations are not as difficult as you might expect.
Thus one electron must be added or removed to discharge a singly charged ion, two electrons must be added or removed to discharge a doubly charged ion, and so on as expected.
The discharge of 1 mole of singly charged ions requires the addition or removal of 1 mole of electrons, the discharge of 1 mole of doubly charged ions requires the addition or removal of 2 moles of electrons, and so on as expected.
All we need to know now is the charge on 1 mole of electrons, because we can work out the charge that flows in a circuit from the current and the time for which it flows. i.e.
.........charge passed.......... =..........
current.......... x..........
time
.........in coulombs (C)..................... in amps (A or Cs-1).... in seconds (s)
It turns out that the charge on 1 mole of electrons = 96,500 coulombs, sometimes known as 1 Faraday. Thus, the discharge of 1 mole of singly charged ions involves 1 Faraday (96,500C), the discharge of 1 mole of doubly charged electrons involves 2 Faradays (193,000C), and so on as expected.
11.15. CONDUCTANCE AND CONDUCTIVITY
11.15.1. The conductance of an electrolyte in aqueous solution is a measure of its ability to conduct electricity. It is the reciprocal of its electrical resistance. It is proportional to the cross-sectional area (a) of electrolyte through which the current is conducted, and inversely proportional to the distance through which the current is conducted (l)
Conductivity is the conductance of a 1m cube of solution measured between two 1m2 plates. In practice the conductance of a 1cm cube is measured between 1cm2 plates. Measurements are made using alternating current so as to prevent the occurrence of electrolysis.
The molar
conductivity of an ion is the conductivity a mole of the ions
would have in aqueous solution at infinite dilution. The units usually
quoted are  -1cm2mol-1.
-1cm2mol-1.
11.15.2. The conductivity of a solution will generally increase with increasing concentration of ions. It will also depend on the mobility of the particular ions in aqueous solution. This, however, will decrease above a certain concentration even for strong electrolytes, because positive ions will interact with negative ions. For weak electrolytes, this occurs at much lower concentrations. This is why molar conductivities are given for infinite dilution.
11.15.3. Moreover, the mobility of a particular ion in aqueous solution will depend on the size of the hydration shell it has to drag around with it. Thus, contrary to what might be expected small, highly charged, ions will tend to have low molar conductivities, because they have a high surface charge density and therefore have strongly attracted hydration shells.
Hydrogen and hydroxide
ions have much greater molar conductivities than might be expected.
This is accounted for in terms of making and breaking of bonds and of
hydrogen bonding between water molecules. The ions do not actually move
through the solution.
11.15.4. The end point
in acid/base titrations can be determined using conductivity
measurements. This is particularly useful in titrations of weak acids
with weak bases, when normal indicator methods cannot be used (section
10.2.7. and 10.2.8.).
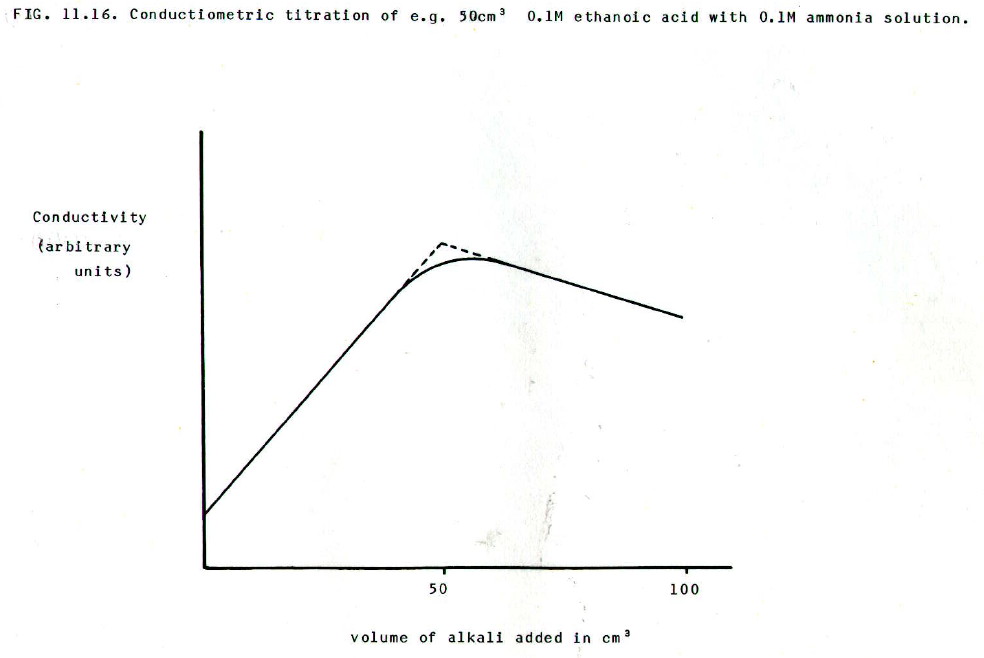
Consider for example the titration of a weak monobasic acid with a weak monoacidic base of equal concentration. At first, the conductivity increases quite rapidly because addition of base to the partially dissociated acid produces a salt which is a strong electrolyte. However, after the end point, the conductivity decreases slowly because the strong electrolyte (salt) is effectively dispersed in weak electrolyte (further weak base).
The results are plotted
(FIG. 11.16) and the two slopes extrapolated, as shown. The equivalence
point is determined from the volume of base added at the intersection
of the two extrapolated lines.
FIG
11-16 (Click on diagram to enlarge on a new web page)
11.16. QUESTIONS
1) Use the following standard electrode potentials to answer the questions below. Do not bother with balanced equations except in part f.
Ce4+/Ce3+ = +1.45V
Cr3+/Cr2+ = -0.41V
Cr2O72-/Cr3+ = +1.33V
Fe3+/Fe2+ = +0.77V
Fe2+/Fe = -0.44V
Sn4+/Sn2+ = +0.15V
Sn2+/Sn = -0.14V
Zn2+/Zn = -0.76V.
i) Explain what would you expect to happen if you placed a piece of iron metal in a solution containing zinc ions.
ii) Explain what you would expect to see if you placed a piece of zinc metal in a solution containing iron 3+ ions.
iii) Rusting involves the oxidation of iron by oxygen from the atmosphere. It occurs in the presence of water. Galvanised iron is iron coated with zinc and it is resistant to rusting. Why?
iv) Predict the likely products when acidified potassium dichromate(VI) is added to: i) aqueous iron(II) chloride, ii) tin, iii) zinc, iv) aqueous cerium(III) chloride.
v) In applying the redox potentials, what assumptions have you made? In other words, how "likely" are the products you have predicted?
vi) Write balanced ionic equations for the reactions you predicted in parts b and d.
2) Where needed, suggest some experiments to test your answer to the question at the end of section 11.5.1.
3) With respect to 2+ ions, metal X is very slightly more electropositive than metal Y. Metal X reacts exothermically with a solution containing Y2+ ions.
i) Write an equation for the reaction between metal X and a solution containing Y2+ ions.
ii) Write a cell diagram for the cell in which this is the cell reaction.
iii) What will be the effect on the EMF of the cell in each of the following circumstances:
a) the concentration of X2+ ions is increased;
b) the concentration of Y2+ ions is increased;
c) the temperature of the cell is increased?
iv) Each of three beakers initially contains a piece of metal X, a piece of metal Y, and a solution which is 1M with respect to both X2+ and Y2+ ions.
a) Comment on the feasibilty of reaction in the beakers.
b) The changes described in part iii) are carried out, one change for each of the three beakers. Comment on the feasibility of reaction in each of the beakers after the changes have been made.
4) In the electrolysis of copper(II) sulphate using graphite electrodes, what mass of copper would be deposited if a current of 0.5 amps flowed for 15 minutes?
5) What shape of graph would you expect if conductivity were plotted against volume of base added in the titration of: i) a strong acid and a strong base, ii) a strong acid and a weak base, iii) a weak acid and a strong base. Explain your reasoning.
Unless otherwise stated, all materials in this web version of chapter 11 are ę 2007 Adrian Faiers MA (Oxon) MCIPR

What 's the connection between a dozen eggs and
a garden mole?

Answer: Not a lot, really, but see Chapter 1