Recommended by:

Top 20 UK science resources,
The Tutor Website
Recommended by:

Rated:

2010
Listed on the science, engineering and technology section of

'providing you with access to the very best Web resources for education and research, evaluated and selected by a network of subject specialists.'
(Please note that intute closed in July 2011)
Section 1: Essentials (physical chemistry)
CHAPTER 12: ENTROPY
(the TOUR continues with the second law of theromodynamics)
NB This chapter has now been
updated to improve browser compatibility.
Please
use the 'send email' link at the top right hand corner of this page to
report any problems.
12.1. BACK ON ROUTE
At the end of chapter 11 we reached a position which enabled us to predict the feasibility of reactions. In this chapter we shall reach the same position by a different route, the route we set out on in chapter 6.
In chapter 6 we decided that enthalpy was a useful concept when observing heat changes during chemical reactions. However, it is not useful for predicting feasibility since there is no regular pattern in the enthalpy change accompanying a feasible reaction. For example, reactions are most often exothermic, but may be endothermic.
To predict feasibility, a new concept is needed: the dissipation of energy, something we realised was happening in the engine compartment of the coach (section 6.2.1.).
12.2. AFTERNOON TEA: ENTROPY (S)
12.2.1. Energy can neither be created nor destroyed (section 7.2.1.), but it can be dissipated. Entropy is a measure of the dissipation of energy, and goes hand in hand with disorder. Moreover, when we observe the entropy changes associated with chemical reactions, a regular pattern does emerge. It is a pattern which applies to any naturally occurring change.
12.2.2. Imagine
yourself in the roadside cafe for afternoon tea, about to
slice into a hot scone. You pick up a warm metal knife and tightly
clasp the handle. Your hand warms up, and the knife handle cools down.
This spontaneous change is clearly described in terms of energy
dissipation.
Initially, heat energy
is concentrated in the knife. In general, heat energy is the kinetic
energy of molecules, atoms, ions etc. resulting from three kinds of
motion shown in FIG. 12.1.
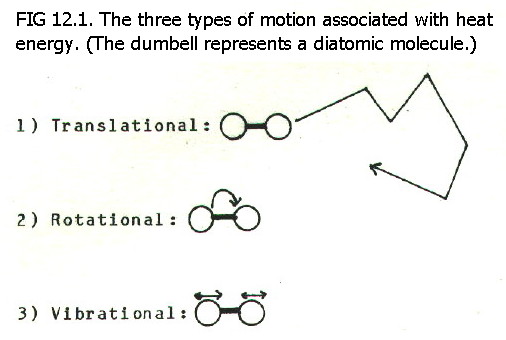
In the case of the metal knife,
the most important of these types is vibration. When the handle is
clasped, vibrating ions at the surface of the handle "jostle" skin
molecules in your hand, and transfer energy to them. The molecules in
the skin, now vibrating more energetically, will similarly transfer
energy deeper into the hand. Moreover, vibrating ions deeper in the
knife transfer energy to the cooling surface of the handle. In this
way, energy is spontaneously dissipated.
It is this transfer of heat energy which makes you describe the knife as warmer than your hand. The random jostling between skin molecules and knife ions is too unlikely to bring about the nett transfer of energy from your hand to the warmer knife for any significant instant of time. The natural direction of change is summarised in the second law of thermodynamics.
12.3. MORE TEA: THE SECOND LAW
12.3.1. The second law of thermodynamics states that any spontaneous change involves an increase in entropy of the universe. In other words, energy is dissipated.
In thermodynamics, spontaneous means able to occur without nett input of energy. It says nothing about rate of change. A spontaneous reaction may occur so slowly that for all practical purposes it does not happen at all. Thermodynamics ultimately gives information about position of equilibrium not about rate. Confusion arises because, in everyday use, spontaneous implies an immediate response.
Universe also needs some explanation. Like infinity, the universe is outside our real experience. In a chemical reaction we tend to observe only the reacting system and its immediate surroundings, such as the reaction vessel. The immediate surroundings are obviously very important.
As for your second cup
of tea, that is cooling down as heat dissipates into the surrounding
air.
12.3.2. Some spontaneous reactions involve a decrease in entropy of the reacting system. At first sight this may appear to be inconsistent with the second law. Thus:
.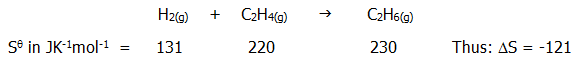
The decrease itself is not surprising. Whereas energy was dissipated over two moles of disordered gas molecules at the start, there is only one mole of gas, occupying only half the volume, at the end of the reaction. So how can the reaction be spontaneous? For the answer we surprisingly need to look at the enthalpy change, but only as an indicator of overall entropy change.
The reaction is exothermic; DH = -93.0kJmol-1. This means that 93kJ of heat energy will be dissipated in the surroundings, increasing their entropy. In this case, the entropy of the surroundings increases by an amount which outweighs the decrease in entropy of the reacting system, and the reaction is spontaneous.
12.3.3. We can now describe, in terms of the second law, how an endothermic reaction can be spontaneous. Heat absorbed from the surroundings decreases the entropy of the surroundings. However, as long as this is outweighed by an increase in entropy of the reacting system, the reaction will be spontaneous. To calculate the total entropy change in a particular case, we need to define entropy in mathematical terms.
12.4. THE FINAL LEG
12.4.1. The heat content of a substance (q) would be expected to give a measure of its entropy: the greater its heat content at a particular temperature, the greater must be its ability to dissipate energy. i.e. S a q. However, heat content obviously depends also on temperature: the higher the temperature of a substance, the greater its heat content. This must be taken into consideration when defining entropy in terms of q. S a 1/T, where T is absolute temperature at which the heat content is measured. In fact, entropy is defined semi-quantitatively in this way:
..........S = q/T
and:
.........DS = Dq/T
When the changes occur at constant pressure Dq = DH and therefore:
.........DS = DH/T.
We are approaching the final destination of the tour. However, note that in the next stages of the journey it is important to remember that energy changes reflect changes in forces of electrostatic attraction (section 6.1.2.). Thus it also follows that the total entropy change reflects changes in forces of attraction in the system and surroundings, as seen by applying the relationship above between DS and DH to the relationships described in the next section..
12.4.2. We have already seen that the total entropy change for a reaction enables us to predict feasibility. Spontaneous reactions are found to involve an increase in:
.......................DSsystem + DSsurroundings.....(= DStotal) [usual form: DStotal = DSsystem + DSsurroundings]
But, the change in entropy of the surroundings is brought about by the enthalpy change in the system, as we saw in section 12.3.2. When the system loses heat, the surroundings gain heat and vice versa. Thus DSsurroundings = -DHsystem/T. Spontaneous reactions therefore involve an increase in:
.......................DSsystem - DHsystem/T.........(= DStotal) [usual form: DStotal = DSsystem - DHsystem/T]
i.e. the value of this function is positive for spontaneous changes. Returning to the example of the hydrogenation of ethene used in section 12.3.2, the entropy change for the surroundings is therefore:

The positive value confirms that we were right to say that the increase in entropy of the surroundings outweighs the decrease in entropy of the system.
However, entropy remains a somewhat esoteric concept. At the end of the tour it proves useful to define a type of energy which enables us to predict feasibility.
12.5. THE DESTINATION: GIBBS FREE ENERGY (G)
12.5.1. The
concept of free energy initially comes about by means of a
little mathematical manipulation. This may not be a very good pedigree,
but the result is surprisingly useful, especially when translated back
into reality.
Spontaneous reactions involve an increase in:
..............DS - DH/T
These two entropy terms can be converted into energy terms by multiplying by T. Thus spontaneous reactions involve an increase in:
..............TDS - DH
i.e. in spontaneous reactions the function is positive, or:
..............DH - TDS is negative.
This energy function is known as the Gibbs free energy function, DG.
i.e.......... DG = DH - TDS (= -TDStotal)
DG is negative for spontaneous reactions i.e. there is a decrease in free energy. Now we have reached the final destination of the chemical energy tour, several things become clear.
12.5.2. First, free energy is the energy free for doing work. It is hardly surprising that this decreases during a spontaneous change since we already know that energy is dissipated during any naturally occuring process.
However, if there has been a decrease in free energy, that amount must have been free to do work. This is certainly the case in electrochemical cells. The free energy can be used to do electrical work and it is found that:
..............DGq = -zFEq
where DGq is the standard free energy change for the cell reaction, z is the number of moles of electrons transferred per mole of reaction, F is the Faraday constant (≡ charge on 1 mole of electrons, 96,487C), and Eq is the standard EMF of the cell. This suggests a useful way of measuring free energy changes.
In living organisms enzymes often couple spontaneous reactions with non-spontaneous reactions, thus using the free energy from the spontaneous reaction to do the work of the non spontaneous reaction (section 25.4.4.).
12.5.3. In addition,the Gibbs free energy change for a reaction can be used to calculate a more understandable quantitative measure of feasibility, the equilibrium constant for a reaction. Again without going into derivations it is found that the relationship between standard free energy change and equilibrium constant is given by:
..............DGq = -RT ln K
Note the similarity with the relationship between Eq and K mentioned in section 11.13.
12.6. A ROUND TRIP
Like all good tours, this one finishes where it started. As at the beginning of chapter 6, we are still unable to say that a reaction occurs because it involves a decrease in energy. However, we can, as suggested then, predict feasibility because spontaneous reactions are observed to involve a decrease in the type of energy we have now described as free energy.
Moreover, the round trip has tied up a lot of loose ends. We can now put our understanding of energy, entropy, equilibrium constant, and EMF in one neat bundle.
12.7. QUESTIONS
1) Do you think the transition from ice → water → steam involves an increase or a decrease in entropy? Explain your reasoning, stating whether or not the transition is spontaneous, and explaining why the system must be heated in order to bring about the change.
2) What do you think is meant by the phrase that a system is thermodynamically unstable but kinetically stable? Include energy considerations in your answer.
3) Spontaneous reactions have negative DG values. Do you think it would be correct to say that the more negative the DG value, the more spontaneous the reaction? If a reaction has a positive DG value, does this mean there will be no forward reaction when the reactants are mixed? CLUE: Your answers should include references to equilibrium.
4) Why was it no surprise to find a strong link between DGq and Eq?
5) Bearing in mind the relationship DS = DH/T, describe how DG reflects changes in electrostatic forces during a reaction.
Unless
otherwise stated, all materials in this web version of chapter 12 are ©
2007 Adrian Faiers MA (Oxon) MCIPR
Animations © 2016 Samuel Faiers

What 's the connection between a dozen eggs and
a garden mole?

Answer: Not a lot, really, but see Chapter 1