Recommended by:
Top
20 UK science resources,
The Tutor Website
Recommended by:

Rated:

2010
Listed on the science,
engineering
and technology section of 
'providing you with access
to the very best Web resources for education and research, evaluated
and selected by a network of subject specialists.'
(Please note that intute closed in July 2011)
Section 2: Inorganic
CHAPTER 14:
PERIODICITY IN PROPERTIES OF
COMPOUNDS OF THE ELEMENTS UP TO ARGON
NB This chapter has now been
updated to improve browser compatibility.
Please
use the 'send email' link at the top right hand corner of this page to
report any problems.
14.1. INTRODUCTION
Across a period, even the changes in chemical behaviour of the compounds can be predicted from the increase in effective nuclear charge. In this chapter we shall deal mainly with hydrides, chlorides, oxides and hydroxides.
For each type of compound we shall be mainly interested in formulae, type of bonding, and behaviour with water. When considering behaviour with water, acid-base properties will be particularly important. Finally, we shall look at the preparation of chlorides.
14.2. STOICHIOMETRY
Before predicting the formulae of the hydrides, chlorides, and oxides (section 4.7.) it is necessary to predict the type of bonding (section 4.4.). The formulae are set out here so that you can check your reasoning once you have read the later sections in this chapter, and worked out the type of bonding in each compound.
| TABLE 14.1. Formulae of hydrides, chlorides and oxides formed by the elements up to argon. | ||||||||
| Period 2 elements | Li | Be | B | C | N | O | F | Ne |
| Hydrides | LiH | BeH2 | B2H6 | CH4.. | NH3 | H2O | HF | ..- |
| Chlorides | LiCl | BeCl2 | BCl3 | CCl4.. | NCl3 | Cl2O.. | ClF | ..- |
| Oxides | Li2O | BeO | B2O3 | CO CO2 |
N2O NO NO2 N2O3 N2O5 |
O2 | OF2 | ..- |
| Period 3 elements | Na | Mg | Al | Si | P | S | Cl | Ar |
| Hydrides | NaH | MgH2 | AlH3 | SiH4.. | PH3 | H2S | HCl | ..- |
| Chlorides | NaCl | MgCl2 | AlCl | SiCl4.. | PCl3 PCl5 |
SCl2 S2Cl2 |
Cl2 | ..- |
| Oxides | Na2O Na2O2 |
MgO | Al2O3 | SiO2 | P4O6 P4O10 |
SO2 SO3 |
Cl2O Cl2O3 Cl2O5 Cl2O7 |
..- |
14.3. HYDRIDES
14.3.1. Bonding in the hydrides shows changes across the period which are largely predictable from the increase in effective nuclear charge. The changes lag slightly behind in the third period because the extra shell reduces attraction of the nucleus for electrons in the outer shell. The relationship between bonding and state is discussed in sections 4.6.1. and 5.2.5.
Thus on the left of the table the low effective nuclear charge means that the elements readily lose electrons and occur as the positive ions in ionic hydrides. They are therefore high melting-point, crystalline solids.
As effective nuclear charge increases across the period, the tendency to lose electrons decreases and they are controlled by the nucleus within covalent bonds. Both B2H6 and (AlH3)n are covalent. However, as the formula implies, aluminium hydride still exists as a solid lattice structure. This may be regarded as remnant of ionic character (section 4.6.2.ii.), though the precise structure is unclear. Even boron hydride exists as a rather unusual dimer, though it is gaseous.
| TABLE 14.2. Bonding and state of hydrides of elements up to argon | |||||||
| Period 2 hydrides | LiH | BeH2 | B2H6 | CH4 | NH3 | H2O | HF |
| Bonding | ionic | ionic | covalent | covalent | covalent | covalent | covalent |
| State at 20 °C | solid | solid | gas | gas | gas | liquid | liquid |
| Period 3 Hydrides | NaH | MgH2 | (AlH3)n | SiH4 | PH3 | H2S | HCl |
| Bonding | ionic | ionic | covalent | covalent | covalent | covalent | covalent |
| State at 20 °C | solid | solid | solid | gas | gas | gas | gas |
By group IV there is no ambiguity. The high effective nuclear charge
holds the electrons in precise covalent bonds with only weak van der
Waals forces between molecules. The strong control which the nuclei
have over the outer electrons ensures that the instantaneous dipoles
which give rise to van der Waals bonding are particularly short-lived.
However, by group V the effective nuclear charge is so high that the covalent bonds are polarised in the opposite direction from the ionic bonding on the left of the table. Moreover, lone pairs on the nitrogen atoms enable hydrogen bonding to occur. the hydrides of both nitrogen and phosphorus are still gaseous at room temperature and pressure, but ammonia is especially easy to liquefy.
In the second period, as well as accounting for the higher than expected melting point and boiling point of ammonia, hydrogen bonding results in both water and hydrogen fluoride being liquids at room temperature. Water even forms a solid lattice at a temperature not far below room temperature. This behaviour could be regarded as ionic character.
In this respect, the same increase in effective nuclear charge is not enough to counteract the extra shell in sulphur and chlorine; thus H2S and HCl are not liquid. However, both are strongly polarised and their reactions with water, discussed below, show the extent of the polarisation.
Summarising, the increasing effective nuclear charge brings about a change from ionic, through covalent with ionic character and simple covalent, to covalent with ionic character showing the opposite polarity from that at the start of the period.
14.3.2. Reactions of the hydrides with water are also largely predictable in terms of the increasing effective nuclear charge. Moreover, these predictions are made easier now that the changes in bond type have been predicted.
i) On the left of the table we have already seen that the high effective nuclear charge results in the formation of ionic hydrides containing metal cations and negatively charged hydride ions.
The very small size of
hydride ions gives them a high surface charge density. Thus when ionic
hydrides are dissolved in water the hydride ions are strongly attracted
to the d+ hydrogen atoms
of water molecules. Moreover, the high effective nuclear charge of
water's oxygen atom, makes a hydrogen ion readily available to form a
covalent bond with the hydride ion (c.f. section 20.5.):
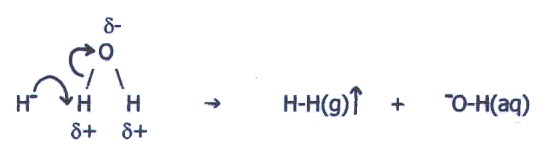
This type of salt
hydrolysis thus produces an alkaline solution.
ii) In the middle of the table the higher effective nuclear charge is similar to that of hydrogen and, as seen above, this leads to formation of strong covalent bonds which are largely unpolarised.
Thus not only do these covalent hydrides not react with water, they do not even dissolve in it appreciably. The electrostatic forces of attraction which could exist between covalent hydride molecules and polarised covalent water molecules, do not compare with those between one polarised water molecule and another (TABLE 4.2. and section 5.2.5.).
In fact, silicon's extra shell relative to carbon, does make it susceptible to slow hydrolysis, for four reasons:
First, the bonding electrons are attracted less strongly, giving the silicon a partial positive charge and making it susceptible to nucleophilic attack by water molecules (section 20.6.).
Second, the larger size of the silicon atom means that there is more room for water molecules to get in and attack it.
Third, there are available 3d-orbitals to accept the incoming electrons during the transition stage. Carbon's outer shell is the second shell and there are no 2d-orbitals. However, contrary to explanations given in many books, this does not mean it has no capacity to form more than four bonds during a transition phase (section 20.5.), it simply makes it more difficult.
Fourth, the relative sizes and effective nuclear charges of carbon, silicon, hydrogen, and oxygen mean that the Si-H bonds are weaker than the C-H bonds, whereas the Si-O bonds are fractionally stronger than the C-O bonds that would form if methane were hydrolysed.
Thus:.... SiH4(g).... +....
4H2O(l)....  ....
Si(OH)4(s)....
+....
4H2(g)
....
Si(OH)4(s)....
+....
4H2(g)
Note that despite anything that may have been said about silicon's extra shell, the effective nuclear charge is still high enough to attract electrons away from an O-H bond and into an Si-O bond. However, the Si-O bond is, in fact, weaker than the O-H bond, but remember that the total reaction involves replacement of 4O-H and 4Si-H bonds by 4Si-O and 4H-H bonds. H-H bonds are considerably stronger than Si-H bonds.
iii) Further increase in effective nuclear charge appears to have somewhat contrary effects: ammonia dissolves to produce alkaline solutions like the hydrides on the left of the table, whereas hydrogen fluoride dissolves to produce acidic solutions. However, these are predicatble consequences of the increasing polarity of the bonds, balanced with decreasing availability of lone pairs for bond formation. Both these factors can be predicted from the increased effective nuclear charge.
Thus in ammonia, the high effective nuclear charge means that the N-H bonds are polarised, exposing nuclear charge on the hydrogen atoms, and concentrating electrons around the nitrogen atom.
This concentration of
electron density not only makes the nitrogen atom susceptible to attack
by the partially positive hydrogen atoms of water (c.f. section 20.9),
it also makes nitrogen's lone pair more available for bond formation
with the hydrogen (c.f. section 20.6.). Even so, nitrogen's high
effective nuclear charge also means that the N-H bond will be fairly
strong.
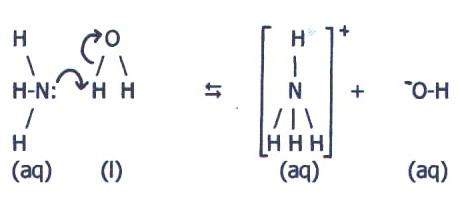
However, oxygen has a higher
effective nuclear charge than nitrogen, and the "fourth" N-H bond is
not as strong as either of the 2H-O bonds in water. Again it is
necessary to consider all the forces of electrostatic attraction
involved, and these include the bonding between water molecules and the
ions formed by the reaction.
Phosphine is considerably less basic than ammonia due to the extra shell in phosphorus.
iv) In groups VI and VII, the effective nuclear charge is even higher. The bonds with hydrogen are therefore even more polarised and, furthermore, the oxygen, sulphur, fluorine and chlorine atoms are all able to pull electrons away from the hydrogen to such an extent, that release of a proton is possible.
As in the reaction of
ammonia with water, hydration of the resultant ions is a determining
factor, but the high effective nuclear charge of oxygen, sulphur,
fluorine, and chlorine does make possible their ability to attract an
extra electron, and thus carry negative charge.
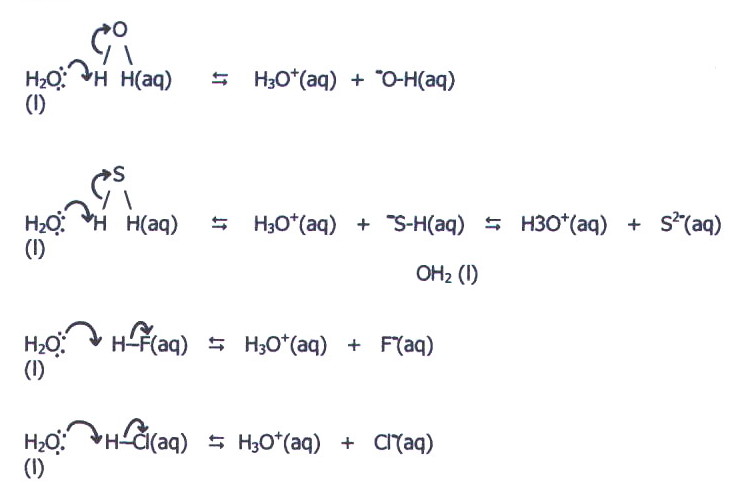
The relative
strengths of these hydrides as acids is confused by two factors:
First, water's reaction with water is a reaction with itself, and the resulting solution contains equal concentrations of hydrogen and hydroxide ions. It is therefore neutral.
Second, for reasons that will be explained in chapter 16, hydrogen fluoride is a much weaker acid than might be expected.
However, there is a useful comparison between hydrogen sulphide and hydrogen chloride. The higher effective nuclear charge of chlorine makes the H-Cl bond stronger than the H-S bond. However, it also makes the chloride ion even smaller than the S2-, and more particularly, much smaller than the -SH ion. This means that the chloride ion attracts water molecules far more strongly, and hydrochloric acid is a strong acid compared with hydrogen sulphide, which is a weak acid.
14.4. CHLORIDES
14.4.1. Bonding in the chlorides of elements up to argon is summarised in table 14.3. below.
| TABLE 14.3. Bonding and state of chlorides of elements up to argon | |||||||
| Period 2 chlorides | LiH | BeCl2 | BCl3 | CCl4 | NCl3 | Cl2O | ClF |
| Bonding | ionic | ionic | covalent | covalent | covalent | covalent | covalent |
| State at 20 °C | solid | solid | gas | liquid | liquid | gas | gas |
| Period 3 chlorides | NaCl | MgCl2 | AlCl3 | SiCl4 | PCl3 PCl5 |
SCl2 S2Cl2 |
HCl |
| Bonding | ionic | ionic | covalent | covalent | covalent ionic |
covalent covalent |
covalent |
| State at 20 °C | solid | solid | solid | liquid | liquid solid |
liquid liquid |
gas |
The changes in bond type across both periods follow the same general pattern as that described for hydrides in the last section, and the reasoning is the same i.e. the changes are predictable from the increases in effective nuclear charge across each period.
The main difference is that chlorine is more electronegative than hydrogen. Thus the "reverse" polarity at the right of the table is less marked. Clearly it does not occur at all at the end of period 3, since chlorine is itself the last element in the period to form a "chloride". Also, it has a higher effective nuclear charge than any of the other elements in the same period. However, the "opposite" polarity does occur in the chlorides of oxygen and fluorine.
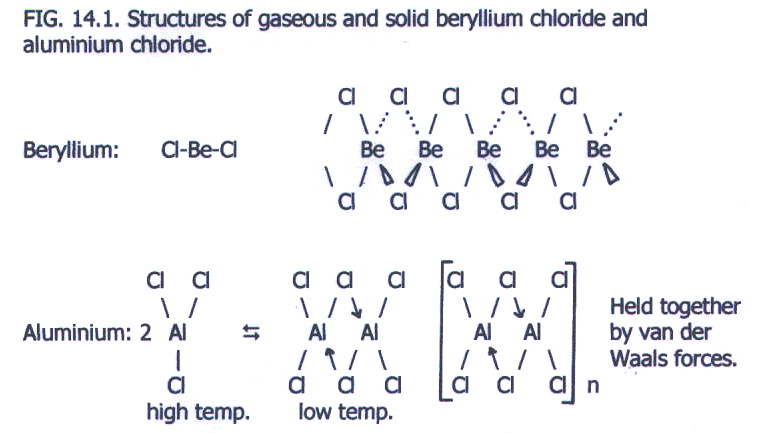
Another difference is that beryllium chloride has a fair degree of covalent character. This is because the chloride ion is larger than the hydride ion and is more polarisable by the beryllium ion, which is small and has a high surface charge density (section 4.6.2.i.) Clearly, aluminium chloride has even more covalent character, enough to be regarded as covalent with ionic character (section 4.6.2.ii).
In the gaseous state,
beryllium chloride consists of linear molecules. In the solid state it
exists as a structure somewhere between an infinite assembly of chains
and an ionic lattice. Aluminium chloride exists as AlCl3
molecules in the gaseous state at high temperature, but Al2Cl6
dimers exist at lower temperatures. Moreover, solid aluminium chloride
exists as the dimers linked to one another by van der Waals forces i.e.
the molecules are definitely more discrete than those in beryllium
chloride.
Apart from phosphorus
pentachloride the rest of the chlorides are discrete covalent
molecules. The simple group IV and V chlorides are liquids, as is
sulphur dichloride. The realtively large size of these molecules allows
for stronger van der Waals forces than are found in Cl2O
and the group VII chlorides. This is because instantaneous dipoles form
more easily in the larger molcules, so it can again be argued that the
liquid state is a last remnant of ionic character. The higher effective
nuclear charge of oxygen, fluorine, and chlorine, together with the
smaller size of the chlorides, makes Cl2O, ClF,
and Cl2 dicrete gaseous moleclues at room
temperature and pressure.
As implied above, phosphorus pentachloride is odd. Table 14.3. indicates that it is ionic, but it does not contain P5+ ions and chloride ions. It comprises [PCl4]+ ions and [PCl6]- ions. This may not be very predictable, but at least it does not severely disturb the basis of our predictions. In the gaseous state phosphorus exists as discrete PCl5 molecules (study question 9.)
14.4.2. Reactions of the chlorides with water follow a predictable pattern, again similar to that of hydrides. In this case, however, the main difference is that chloride ions do not react with water because they are much larger than hydride ions.
i) Thus on the left of the table the ionic chlorides dissolve in water to produce hydrated metal ions and hydrated chloride ions.
E.g..... NaCl(s).... +....
water....  ....
Na+(aq)....
+.... Cl-(aq)
....
Na+(aq)....
+.... Cl-(aq)
ii) By group II, the
higher nuclear charge of the ions makes them considerably smaller. This
combined with their doubly positive charge gives them a high surface
charge density and they are able to attract lone pairs from the oxygen
atoms of water into covalent bonds. Moreover, the effective nuclear
charge of the ion is great enough to pull electrons away from one of
the O-H bonds and allow release of a hydrogen ion:
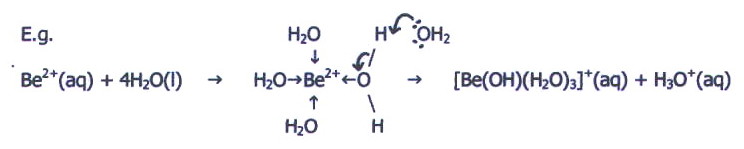
This type of salt
hydrolysis produces an acidic solution. The extra shell in
magnesium makes it larger than beryllium, thus its ion is not so
extensively hydrolysed as that of beryllium.
iii) Group III chlorides are readily hydrolysed. As already seen, the high effective nuclear charge results in covalent bonding. However, chlorine's effective nuclear charge is higher still, and the bonds are therefore polarised, exposing nuclear charge on the central atoms.
This makes them susceptible to nucleophilic attack by water molecules (sections 20.5. and 20.6.). The high effective nuclear charge of boron and aluminium attracts lone pairs from water into strong covalent bonds and away from the O-H bonds thus releasing a proton. The planar shape of the molecules, and the available 2p orbital makes the attacking process particularly easy:
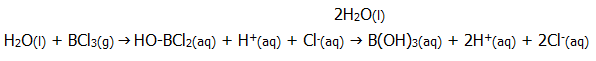
The final product is moderately soluble in water, and itself weakly acidic (section 14.5.2.).
Owing to the extra shell in aluminium, its outer shell is further from the nucleus and it therefore more readily loses electrons and forms positive ions. Thus during the hydrolysis of aluminium chloride, the compound takes on more ionic character and the solution contains hydrated Al3+ ions. However, the high surface charge density of the ions results in salt hydrolysis (section 14.4.2.ii) producing an acidic solution. The hydrolysis is not simple (section 13.9.4.), and there is a decreasing concentration of ions with one, two, and three molecules of water split.
iv) By group IV, the effective nuclear charge is yet higher and we would therefore predict that the covalent chlorides are even more extensively hydrolysed, producing acidic solutions. Silicon tetrachloride does behave as expected.
However, carbon is so small, we might predict that the four bulky chlorine atoms would physically prevent (sterically hinder) water molecules from reaching the central atom. Moreover, unlike silicon, carbon has no available d-orbitals to accept the incoming water electrons during the transition stage. Remember, this in itself does not make formation of the transition stage impossible, but it would be expected to make it even less likely (sections 14.3.2.ii. and 20.5.).
In fact, the combined result of these two effects is that tetrachlormethane is not hydrolysed by water. Interestingly, if it were, the total electrostatic attraction in the products would be greater than in the reactants. In other words, the equilibrium lies in favour of product formation, but the reaction is so slow as not to occur in practice.
If this is true, it should be possible for tetrachloromethane to be hydrolysed at high temperatures, where molecules collide with sufficient vigour for the water molecules to reach the central carbon atom. This is, indeed the case.
More traditionally, hydrolysis of tetrachloromethane is said to be thermodynamically favourable but kinetically unfavourable. The thermodynamic prediction is made by looking at bond energies in the reactants and products, and the kinetic prediction is made by looking at the activation energy.
But, as we have have just seen, it also seems possible to make both predictions by considering only the electrostatic (and steric) arguments. However, the predictions can be checked against energy (enthalpy) changes and activation energies. Moreover, when enthalpy changes and activation energies are used as the basis for predictions, they themselves can be predicted from electrostatic and steric arguments.
It is beginning to appear that the energetic (enthalpy) approach and the electrostatic approach are really the same thing. It is time to bring all the mental processes to a conscious level.
v) Summarising the electrostatic approach vs. the energetic approach:
The energetic approach and the electrostatic approach are the same thing. The difference is the starting point in the arguments. The energetic approach starts with enthalpy values which are determined from experiments (c.f statement in section 4.1.1.). The electrostatic approach starts with electrostatic forces (and steric effects) as described by models of interacting atoms, molecules, ions, etc. (c.f. section 2.1.2. and chapter 20).
Thus, it is possible either
i) to base predictions on experimentally determined energy values and explain those values in terms of the model (energetic approach)
or
ii) to base predictions on models and check them against experimentally determined energy values (electrostatic approach). The models are, of course, themselves based on observation and experiment.
At this level and well beyond, a great deal of confusion arises because this distinction is not made. It is the models which describe the more fundamental and more detailed properties. Using the models, enthalpy changes and activation enthalpies are not only predicted from electrostatic and steric arguments, but they are also explained (section 2.1.2.).
It may seem possible to predict electrostatic and steric arguments from enthalpy changes and activation enthalpies, but this is a different process. It is using experimental results to help in the correct application of the model. This itself is subtly different from using experiments to test the "correctness" of a model, an important part of scientific method:

Thus, in the energetic approach:
- It is usually enthalpy which is considered (section 14.3.2.iii.).
- Predictions about equilibrium (thermodynamic predictions) are made by considering the energy difference between products and reactants.
- Predictions about rate (kinetic predictions) are made by considering the energy difference between the reactants and the transition state (activation energy), for both forward and backward reactions.
- Predictions about both types of energy difference are made by considering electrostatic forces; steric effects are also considered.
In the electrostatic approach:
- Predictions about rate are made by looking at electrostatic forces which occur between reactants, and the effect they have on the ease with which transition stages are reached. Steric effects are also considered.
- Predictions about equilibrium are made by looking at the difference in total electrostatic attraction between reactants and products.
- Bond energies are used to check predictions.
In both cases:
- Entropy changes must be taken into consideration when predictions are not clear-cut (chapter 12). Even then, energy and electrostatic forces take their correct positions in the arguments (sections 6.1.2. and 12.4.1.), though it is impractical to present the complete picture in every instance. For convenience, the concept of free energy is used in a way which by-passes a rigorous analysis of each individual situation.
FIG.14.2: Summary: Both diagram i) and diagram ii) summarise the two approaches. In both diagrams S1 represents starting points for the energetic approach, and S2 is the starting point for the electrostatic approach. However, diagram i) emphasises the energetic approach, and diagram ii) emphasises the electrostatic approach.
i) Emphasis on energy:
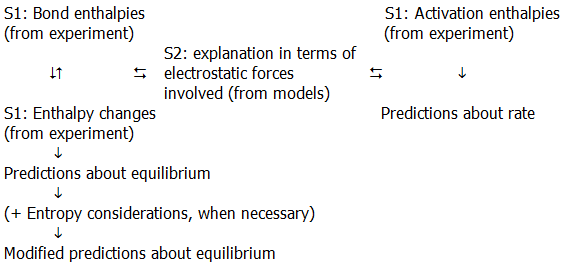
ii) Emphasis on electrostatic forces:
S2: Consideration of
electrostatic forces (from models)
Checked against bond enthalpies
(S1, from experiment)
.............................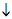
Predictions about forward and
backward rates
Checked against activation
enthalpies (S1, from experiment)
.............................
Predictions about equilibrium
.............................
Checked against enthalpy changes
(S1, from experiment)
.............................
(+ Entropy considerations when
necessary)
.............................
Modified predictions about
equilibrium
Thus we finish this section where we started. The two approaches are really the same. The distinction between the two starting points is intentionally over-emphasised throughout this book because, although clearly adopted at a sub-conscious level by experienced chemists, it is rarely recognised by new students. This leads to a great deal of confusion, and to some bizarre ideas about energy (section 6.1.1.).
It may be true that energy plays an important role in the construction of atomic and molecular models, but its importance in the model itself must not be over-emphasised (sections 2.3.3., 4.1.1., 4.4.5., and 4.8.).
vi) The remaining chlorides (groups V to VII) are all hydrolysed, though there is more to say than that.
By group V the effective nuclear charge has increased such that nitrogen's is about the same as that of chlorine, as measured on Pauling's electronegativity scale. This measures the relative tendency of an atom to attract a bonding pair of electrons on an arbitrary scale of 0 to 4. Both nitrogen and chlorine have electronegativities of 3.
The N-Cl bonds are therfore largely unpolarised and attack on the nitrogen by the oxygen atoms of water is therefore not favoured. Moreover, the lack of available d-orbitals in nitrogen makes its acceptance of lone pairs from attacking water molecules even less likely.
However, the nitrogen
has an available lone pair for bond formation with water's hydrogen
atoms. It is therefore collisions with this orientation that favour
reaction:
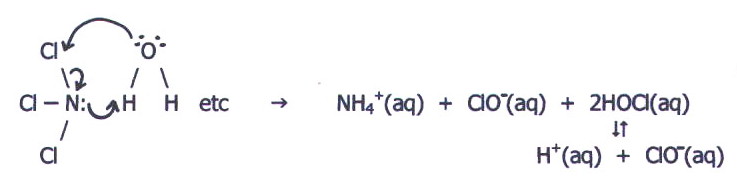
Cl2O and
Cl-F undergo hydrolysis in a similar way and for similar reasons. The
other chlorides of oxygen also undergo hydrolysis.
In the next period, the
extra shell makes phosphorus and sulphur less electronegative than
nitrogen and oxygen, and clearly less electronegative than chlorine.
Thus, both PCl3 and SCl2
have exposed nuclear charge on the central atoms. Moreover, both have
available 3d-orbitals to accept the incoming water electrons. It is
predictable, therefore, that PCl3 is hydrolysed
in the following way:
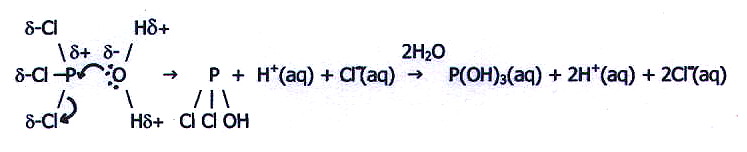
However, the higher effective
nuclear charge of sulphur compared with phosphorus, together with its
two lone pairs, compared with one on phosphorus, means that SCl2
probably reacts more like Cl2O, despite the
available d-orbitals. Which of the two mechanisms occurs when chlorine
reacts with water is somewhat hypothetical:
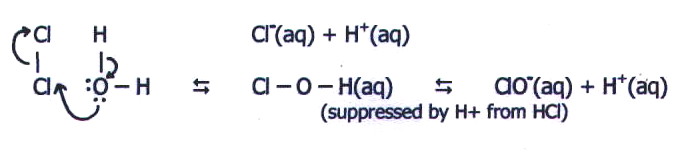
14.5.
OXIDES AND
HYDROXIDES
14.5.1. Bonding in the oxides of elements up to argon should by now be fairly easily predicted. It is summarised in table 14.4. below.
| TABLE 14.4. Bonding, state, and acid-base properties of oxides of elements up to argon. | |||||||
| Period 2 oxides | Li2O | BeO | B2O3 | CO2/ (CO) |
N2O5 N2O3 NO2/ NO N2O |
O2 | OF2 |
| Bonding | ionic | giant | covalent | covalent | covalent | covalent | covalent |
| State at 20 °C | solid | solid | solid | gas | N2O5 solid, rest gases | gas | gas |
| Acid/base reaction | basic | ampho | acidic | acidic/ (neutral) |
acidic/ ampho |
neutral | (acidic) |
| Period 3 oxrides | Na2O | MgO | Al2O3 | SiO2 | P4O10/ (P4O6) |
SO3/ S2O2 |
Cl2O7 Cl2O |
| Bonding | ionic | ionic | giant | covalent | covalent |
covalent |
covalent |
| State at 20 °C | solid | solid | solid | solid | solid | liquid/ gas |
liquid/ gas |
| Acid/base reaction | basic | basic | ampho | acidic | acidic | acidic | acidic |
As might be predicted, the two oxides whose bonding is referred to as giant, are intermediate between ionic lattices and giant covalent structures. Both err slightly on the side of being ionic with a high degree of covalent character.
14.5.2. The reactions of the oxides with water is once more predictable from changes in effective nuclear charge across the periods. As can be seen from table 14.4. the important aspect of the behaviour is its acid/base character.
B, the elements have a
low effective nuclear charge, and the oxides are ionic. Ionic oxides
produce oxide ions in aqueous solution, which, on account of their high
surface charge density, are hydrolysed to produce an alkaline solution
(c.f. section 14.3.2.i.).
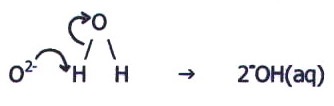
ii) On the right of the
table, the elements have a high effective nuclear charge and
form covalent oxides. However, with the exception of fluorine (and, of
course, oxygen), all the elements have a lower effective nuclear charge
than oxygen and are consequently more electropositive than oxygen. It
can thus be predicted that the bonds with oxygen will be polarised such
that water will nucleophilically attack the non-oxygen atom in the
oxides (sections 20.5. and 20.6.).
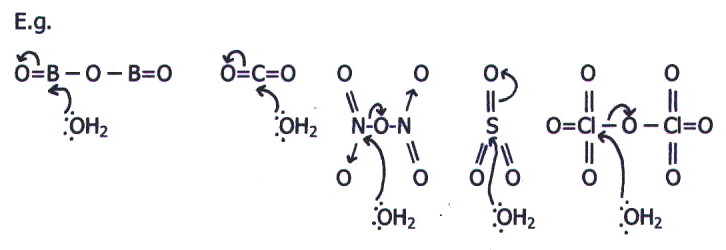
When there is more than one
oxide, lower oxides might not show quite such acidic behaviour. Thus
carbon monoxide is neutral and insoluble in water. N2O
and NO are equally neutral and insoluble. i.e. the lower the degree of
covalency, a property associated with high effective nuclear charge,
the lower the exhibition of acidity, another property associated with
high effective nuclear charge. This is a nice link, but only a clue as
to what is going on.
The relevant point is that the greater the number of electronegative oxygen atoms attached to the non-oxygen atom, the greater the exposure of nuclear charge. This makes the non-oxygen atom more susceptible to nucleophilic attack by the water molecules.
For elements on the right of the third period, all the important oxides are acidic because the extra shell makes it easier for the atoms to lose control of bonding electrons to the electronegative oxygen. However, silicon dioxide does not dissolve in water because its macromolecular lattice is far too strong.
Fluorine's extremely high effective nuclear charge makes it the most electronegative element. The bonds in OF2 are polarised d+O-Fd-, and the oxide is generally regarded as neutral and insoluble in water. However it is slowly hydrolysed:
F2O(g)..... + H2O(l)........ ........
2HF(aq)..... +..... O2(g)
........
2HF(aq)..... +..... O2(g)
...................................................
.................................2H+(aq)..... +.....
2F-(aq)
Moreover, owing to the high effective nuclear charge of chlorine, it might be predicted that exposure of nuclear charge in the lowest oxide of chlorine, Cl2O, is not sufficient for it to undergo nucleophilic attack. This has already been discussed in section 14.4.2.vi., but note that the oxide is still acidic.
iii) The production of acidic solutions by hydrolysis of oxides of elements on the right of the table, is itself predictable from their high effective nuclear charge. The high efective nuclear charge not only pulls the water lone pairs into bond formation, but also helps the oxygen atoms in the water molecules to attract electrons from its O-H bonds, thus releasing protons.
The immediate products of the hydrolysis reactions are "hydroxides", though the term hydroxyacid is more relevant (section 14.4.3.).
iv) Inbetween the basic oxides and the acidic oxides, come amphoteric oxides. The property is due to intermediate effective nuclear charge: note that the changeover occurs later in the third period owing to the extra shell.
Neither beryllium oxide nor aluminium oxide reacts with water, but both can be encouraged by acids to show basic behaviour, and by alkali to show acidic behaviour, though in the case of aluminium oxide, only freshly prepared samples dissolve in either acids or bases:
BeO(s)..... +.....
2H+(aq).....  .....
Be2+(aq).....
+.....
H2O(l)
.....
Be2+(aq).....
+.....
H2O(l)
.......................................................................................basic behaviour
Al2O3(s)..... +..... 6H+(aq).....  .....
2Al3+(aq).....
+.....
3H2O(l)
.....
2Al3+(aq).....
+.....
3H2O(l)
BeO(s)..... +..... 2-OH(aq)..... +..... H2O(l).....  .....
[Be(OH)4]2-
.....
[Be(OH)4]2-
.......................................................................................acidic behaviour
Al2O3(s)..... +..... 2-OH(aq)..... +..... 3H2O(l).....  .....
2[Al(OH)4]-(aq)
.....
2[Al(OH)4]-(aq)
14.5.3. The changes across the period in acid/base behaviour of the hydroxides are a direct function of increasing effective nuclear charge. This can be seen in terms of its effect on the X-O-H bonds:
i) On the left of the table the low effective nuclear charge means that the elements tend to lose electrons and form positive ions, as already seen. The hydroxides thus tend to consist of positive metal ions and negative hydroxide ions i.e. the X-OH bond is ionic: X+ -OH. In water, these hydroxides therefore produce hydroxide ions and the solutions are alkaline:
E.g. ..NaOH(s).... +....
water....  ....
Na+(aq)....
+.... -OH(aq)
....
Na+(aq)....
+.... -OH(aq)
ii) On the right of the table the high effective nuclear charge helps the electronegative oxygen atom pull electrons away from the O-H bond and onto the oxygen, with release of a proton. This gives an acidic solution:
E.g... H-O-Cl(aq).... +....
water....  ....
H+(aq)....
+.... -OCl(aq)
....
H+(aq)....
+.... -OCl(aq)
See also section 14.5.3.iv. below.
iii) Inbetween the basic and acidic hydroxides the intermediate effective nuclear charge X makes the hydroxides amphoteric E.g. aluminium hydroxide section 13.9.4.).
iv) The acidic hydroxides are normally referred to as hydroxyacids. They are formed by hydrolysis of acidic oxides by water (or alkali followed by acid in the case of silicon dioxide):
B2O3...........  .....
H3BO3
.....
H3BO3
CO2.............  .....
H2CO3
.....
H2CO3
N2O3...........  .....
HNO2
.....
HNO2
N2O4
(NO2).. .....
HNO2..
+.. HNO3
.....
HNO2..
+.. HNO3
N2O5...........  .....
HNO3
.....
HNO3
SiO2............  .....
H4SiO4
.....
H4SiO4
P4O6...........  .....
H3PO3.. warm with water..
.....
H3PO3.. warm with water..  ..
PH4+.. +..
H2PO4-
..
PH4+.. +..
H2PO4-
P4O10.........  .....
HPO3..
warm with water..
.....
HPO3..
warm with water..  ..
H3PO4
..
H3PO4
...........................
(H3PO4 dehydration.. ..
HPO3 + H4PO7)
..
HPO3 + H4PO7)
SO2............  .....
H2SO3
.....
H2SO3
SO3...........  .....
H2SO4
.....
H2SO4
Cl2O..........  .....
HClO
.....
HClO
ClO2..........  .....
HClO2..
+.. HClO3
.....
HClO2..
+.. HClO3
Cl2O3........  .....
HClO2
.....
HClO2
Cl2O5........  .....
HClO3
.....
HClO3
Cl2O7........  .....
HClO4
.....
HClO4
As well as being
determined by the effective nuclear charge (and hence
electronegativity) of the non-oxygen atom, acid strength of these
hydroxyacids is also determined by the extent to which negative charge
in the resultant anion can be spread out by delocalisation. Thus acid
strength decreases in the order chloric(VII) acid > chloric(V)
acid > chloric(III) acid > chloric(I) acid, because the
resultant anions are decreasingly stabilised in this order:
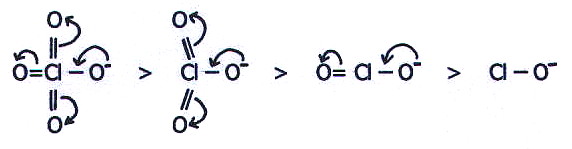
14.6. PREPARATION OF
CHLORIDES
The idea of this section is not to give detailed methods of preparation, but to show that even methods of preparation can be predicted. Most chlorides can be prepared by direct combination of the element with chlorine (section 16.2.5.vi.), though this is not always the most convenient method. Moreover, when there is more than one chloride of a particular element, different techniques may be essential.
Thus direct combination may be conveniently used to prepare BCl3, Al2Cl6, SiCl4, PCl3, PCl5 (excess chlorine), S2Cl2, SCl2 or SCl4 by controlling the flow of chlorine, and ClF. The method for most of these chlorides is to pass dry chlorine over the heated element in a silica tube.
However, to produce chlorides of the group I and II elements, it is far more convenient to titrate hydrochloric acid with the oxides or hydroxides. This is partly because these reactions are far more controllable, and partly because the elements at either end of the table are predictably more readily available as compounds.
The small size and high effective nuclear charge in carbon and nitrogen means that bonding is particularly strong in both these elements. Reaction of carbon and nitrogen is therefore difficult, and nitrogen does not react with chlorine at all. Both tetrachloromethane and nitrogen trichloride are thus produced by reaction of the hydrides with chlorine. Nitrogen trichloride is an explosive, yellow, oil.
14.7. QUESTIONS
1) Which of the factors governing the hydrolysis of SiH4 (section 14.3.2.ii.) is kinetic, and which thermodynamic?
2) Explain the relative strengths of the Si-H, O-H, Si-O, and H-H bonds described in section 14.3.2.ii. in terms of the relative atomic sizes and relative effective nuclear charges of the elements involved. How do these relative strengths relate to bond energies?
3) Write equations showing electron movements for hypothetical reactions in which a) ammonia reacts like HCl in water and b) HCl reacts like ammonia in water. Explain why these reactions do not occur?
4) Explain in more detail the statement at the end of section 14.3.2.iii: 'Phosphine is considerably less basic than ammonia due to the extra shell in phosphorus'.
5) The dissociation of water is mentioned in section 14.3.2.iv. If you had to choose one of the following descriptions of the dissociation, explain which which you would choose and why: i) water acts like ammonia by pulling a proton off a(nother) water molecule or ii) water acts like hydrogen sulphide by releasing a proton to a(nother) water molecule.
6) Hydrogen sulphide can lose two protons in aqueous solution, but water does not. Describe the basis of this difference in behaviour.
7) Describe the formation of Al2Cl6 dimers from AlCl3 monomers in terms of orbital hybridisation.
8) Predict and draw the shapes of BCl3, CCl4, PCl3, SCl2, and ClF molecules.
9) Predict draw the shapes of [PCl4]+ and [PCl6]- ions, plus the shape of the PCl5 molecule. What do you know about nitrogen that would enable you to predict that it is unlikely to form NCl5. Explain your reasoning.
10) What is the role of boron's vacant p-orbital in the hydrolysis of boron trichloride? Draw the structure of the intermediate in the replacement of the first chlorine atom, and suggest how boron's orbitals are hybridised (section 4.8.) at this stage. Write a balanced equation for the overall hydrolysis.
11) Write balanced equations for the complete hydrolysis of NCl3, PCl3, SCl2, Cl2O, and ClF.
12) Describe how changes in bonding within oxides in periods 2 and 3 is related to a) the increase in effective nuclear charge across both periods, and b) the extra shell in period 3.
13) Write balanced equations for the reactions of Na2O and MgO with water.
14) In the second period, the group II oxide is amphoteric. In the third period, the group II oxide is basic, and it is not until group III that an amphoteric oxide appears. Explain this difference in terms of the extra shell in period 3 elements.
15) Combining data in sections 14.4.2 and 14.4.3.iv. write balanced equations for the hydrolysis of the acidic oxides. Which of these reactions are disproportionation reactions (section 11.3.4.).
Unless otherwise stated, all materials in this web version of chapter 14 are © 2007 Adrian Faiers MA (Oxon) MCIPR

What 's the connection between a dozen eggs and
a garden mole?

Answer: Not a lot, really, but see Chapter 1