Recommended by:

Top 20 UK science resources,
The Tutor Website
Recommended by:

Rated:

2010
Listed on the science, engineering and technology section of

'providing you with access to the very best Web resources for education and research, evaluated and selected by a network of subject specialists.'
(Please note that intute closed in July 2011)
Section 2: Inorganic
CHAPTER 17: GROUP IV
NB This chapter has now been
updated to improve browser compatibility.
Please
use the 'send email' link at the top right hand corner of this page to
report any problems.
17.1. INTRODUCTION
The low effective nuclear charge in groups I and II, and the high effective nuclear charge in group VII dominated the chemistry of these groups. It outweighed the effect of increasing number of shells down the groups.
In contrast, group IV is in the middle of the periodic table and elements within group IV have an intermediate effective nuclear charge, which is dominated by the increasing number of shells down the group. The effective nuclear charge is such, that the increasing number of shells down the group takes the elements from being predominantly electronegative at the top of the group, to predominantly electropositive at the bottom of the group.
This is a change from predominantly non-metallic behaviour to predominantly metallic behaviour, and it could be argued that the key difference between group IV changes and changes down the groups discussed in chapters 15 and 16 is that in group IV they are merely more noticeable. For example, it is easier to see the difference between a non-metal and a metal, than it is to see the difference between an electropositive metal and an even more electropositive metal.
In support of this argument, it is interesting to note that down group I, Pauling's electronegativity values (section 14.4.2.iv.) decrease by 30% and down group IV they decrease by roughly the same amount, 28%. Moreover, if the head element, carbon, is removed from the reckoning, all the remaining elements in group IV have the same electronegativity value, 1.8. In contrast, there is a decrease from 0.9 to 0.7 between sodium and caesium in group I.
Some of the more interesting changes in properties of the elements and compounds are discussed below. As in previous chapters, the order in which the properties are presented is intended to be a logical progression from the fundamental basis of changes down the group, i.e. increasing number of shells. Every now and again, the text refers right back to this underlying change in order to stress its importance as the starting point in all detailed descriptions of changes in properties down a group.
17.2. THE ELEMENTS
17.2.1. Bonding and state
i) Allotropes: Both carbon and tin exist in more than one form. The different forms of an element are known as allotropes. The existence of these allotropes is in many ways a reflection of the low effective nuclear charge in group IV, since one form is always more metallic, and another more non-metallic.
The allotropes of carbon have already been discussed in section 5.2.3. Note that one form, diamond, is more non-metallic, and the other, graphite, is more metallic.
Tin exists in three forms, grey, white, and brittle. Grey tin is metastable. It has a diamond-type structure and is the most non-metallic form. The common form is white tin which has a distorted close-packed/body-centred cubic structure (section 5.2.4a&c.), and in which the bonding is metallic.

When allotropes can undergo reversible transformation from one form to another, at a definite transition temperature, they are known as enantiotropes, not to be confused with enantiomers (section 24.3.3.).
ii) Non-metallic vs. metallic bonding. It is true that even carbon and silicon do not exist as discrete molecules, and that the macromolecular structure is itself some indication of metallic character. Even so, the closeness of the bonding electrons to the nuclei does make the bonding predominantly covalent and particularly strong, especially in carbon.
As the outer electrons become further from the attractive influence of the nucleus down the group, the bonding not only becomes weaker, but there is a definite increase in metallic character.
In other words, as the bonding electrons become further from the influence of the nuclei, the likelihood of finding them restricted to definite covalent bonds decreases. The bonding orbitals become less discrete until in white tin and in lead there is one metallic bonding orbital which permeates the whole lattice.
The metallic character of graphite (shiny appearance and ability to conduct electrons) is a rather special case. The metallic characteristics of silicon and germanium are more genuine examples of the increasing metallic character. Both these elements have a diamond-type structure, a structure which is very unmetallic in carbon.
Thus both germanium and silicon are semi-conductors. Moreover, silicon can exist as a dark grey shiny solid (or a brown solid, depending on crystal size) and germanium has a grey metallic appearance. Germanium is regarded as a metalloid.
The changes in bond
type are reflected by the changes in melting point and boiling point.
Thus there is a decrease in m.p. and b.p from carbon to silicon as
covalent bond strength decreases and less vigorous vibration of the
atoms is needed to overcome the attractive forces involved. The decrease in covalent bond
strength is due to the increased distance between the nuclei and the
covalent bonding electrons.
(Click anywhere on diagram to
enlarge on a new web page) 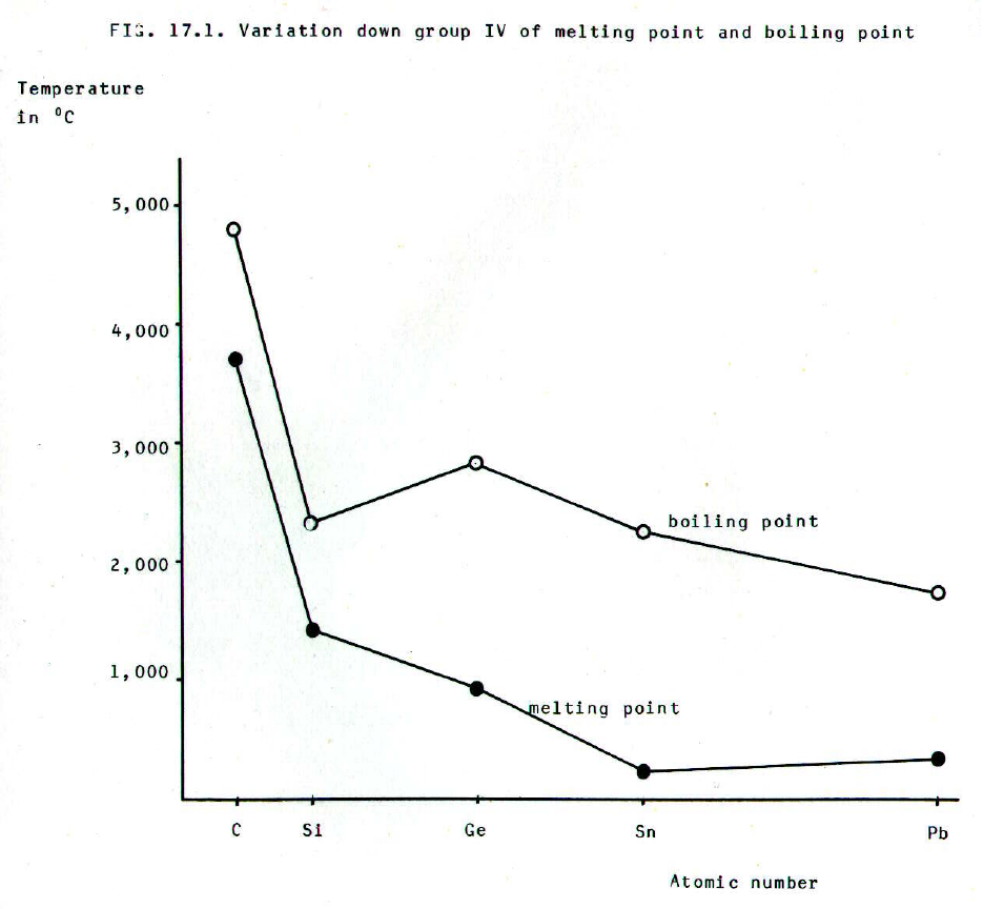
The melting point further
decreases to germanium and then to tin as the bonding electrons become
yet further from the nuclei. (The change to metallic bonding is not a
significant factor in this respect.) The slight increase in melting
point between tin and lead is due to the increased effective nuclear
charge associated with the filling of f-orbitals and their relatively
low screening effect (section 13.2.). Morover, lead has a cubic
close-packed structure (section 5.2.4c.) which is tighter than the
body-centred cubic arrangement in tin.
Boiling point shows a slight increase between silicon and germanium. This is an indication of the change from covalent to metallic bonding. When silicon melts, the covalent bonds are broken and little further breaking of bonds is required for boiling to occur. However, when germanium melts, metallic bonding exists in the liquid and this must be broken for melting to occur.
17.3. FORMATION OF COMPOUNDS
17.3.1. Covalent vs. ionic bonding: Just as the intermediate effective nuclear charge in group IV and the increasing number of shells down the group lead to an increase in metallic bonding in the elements, so also they lead to an increasing tendency to form positive ions in the compounds.
At the top of the
group, the closeness of the outer electrons to the nucleus (especially
in carbon) enables them to be controlled in strong covalent bonds.
Carbon's attraction for electrons is so great, that it can even form
the negative dicarbide ion, C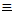 C-.
At the bottom of the group, the greater distance of the outer electrons
from the nucleus allows them to be more easily lost. However, Sn4+
and Pb4+ ions exist only in complexes. As will
be discussed in the next section, there is a greater tendency to form
2+ ions. Germanium forms only a 2+ ion, even in complexes.
C-.
At the bottom of the group, the greater distance of the outer electrons
from the nucleus allows them to be more easily lost. However, Sn4+
and Pb4+ ions exist only in complexes. As will
be discussed in the next section, there is a greater tendency to form
2+ ions. Germanium forms only a 2+ ion, even in complexes.
17.3.2. i) 4-valency vs. 2-valency: Down the group there is a decreasing tendency to form 4-valent compounds and an increasing tendency to form 2-valent compounds. Since formation of 2-valent compounds excludes the pair of outer s-electrons from bond formation, the tendency is known as the inert pair effect. Note that the inert pair is an effect, not a cause.
A more detailed description of the underlying basis for the effect is that the total electrostatic forces of attraction on forming the two extra bonds becomes less able to overcome the attractive forces in the bonds which must be broken. This is reflected by the associated energy changes.
ii) The case of covalent bond formation is best considered using an example such as hydride formation:
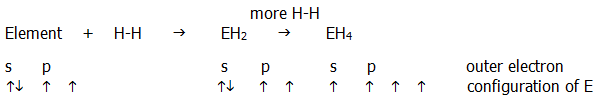
It can be seen that an s-electron must be pulled further from the nucleus of E into a p-orbital, and far more importantly, an H-H bond must be broken. Since the E-H bonding electrons become further from the E nucleus as the group is descended, the bonding forces of attraction become weaker. Thus formation of two extra E-H bonds becomes less likely to outweigh the forces which must be overcome on promotion of electrons from the s-orbital and the breaking H-H bond. This is both a kinetic and a thermodynamic effect, and it is reflected by the relevant activation energies as well as bond and promotion energies.
The
nett change in the relative stabilities of the 4-covalency and
2-covalency is summarised in FIG. 17.2. Note that the changeover point
is between germanium and tin.
(Click anywhere on diagram to
enlarge on a new web page) 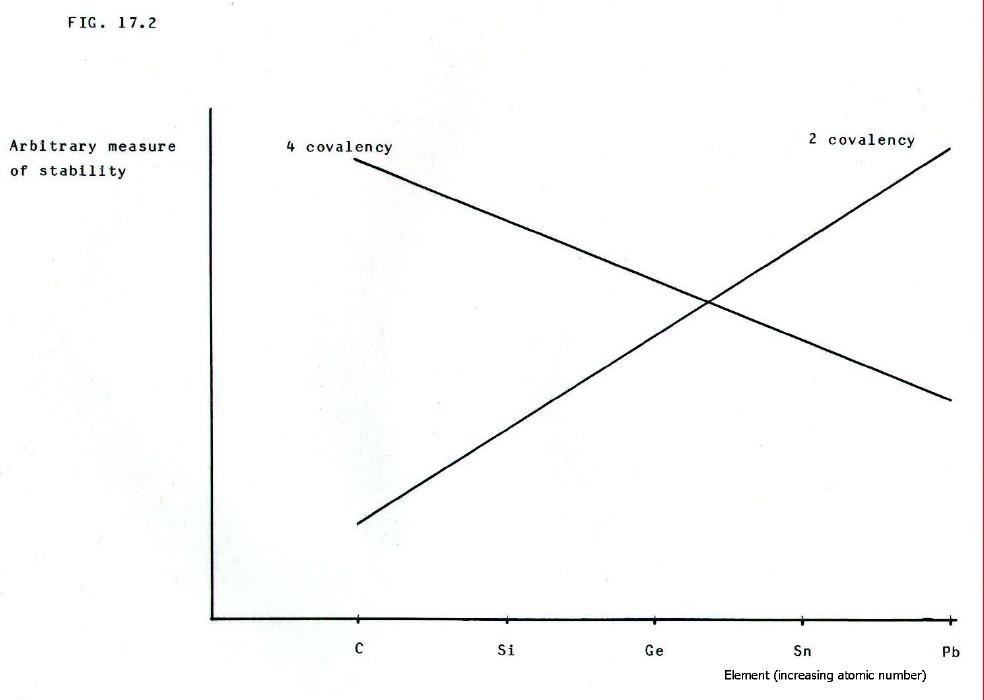
iii) The case of
electrovalent bond formation is only relevant from germanium
downwards. The key change is the removal of another two electrons to
form a 4+ ion from a 2+ ion. Germanium forms only a 2+ ion because its
outer shell is too close to the nucleus for further electrons to be
totally removed.
However, tin and lead can form both 4+ and 2+ ions. The tendency to form the 2+ rather than the 4+ ion increases between tin and lead, partly because the 3rd and 4th electrons become slightly more difficult to remove. This results from the increased effective nuclear charge associated with filling of the f-orbitals, and their relatively low screening effect, already mentioned above.
Moreover,
the extra forces of attraction on formation of a 4+ ion from a 2+ ion
are the stronger forces of attraction in the ionic lattice (or the
extra attraction for water molecules in aqueous solution - note that
all these ions are hydrolysed in aqueous solution
(section 14.4.2.ii.)). In either case the extra forces result from the
smaller size and higher charge of the 4+ ion giving it a greater
surface charge density.
(Click anywhere on diagram to
enlarge on a new web page) 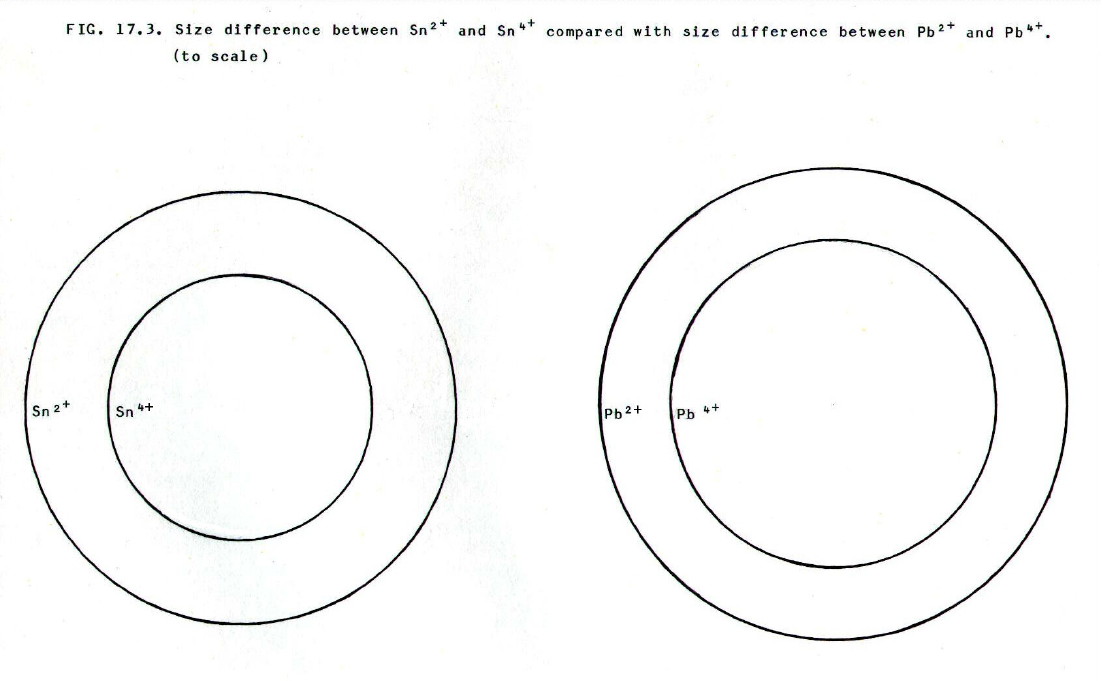
Since the extra shell makes lead
larger than tin, the reduction in size on forming Pb4+
from Pb2+ is less marked than the reduction in
size when the smaller Sn4+ion forms from Sn2+.
Thus the increase in surface charge density is also less marked, and
the consequent increase in attraction for negative ions in the lattice
(or water molecules in solution) is correspondingly less marked i.e.
less able to provide the driving force for removal of two extra
electrons.
The nett change in
relative stabilities of the 4-electrovalency and 2-electrovalency is
summarised in FIG. 17.4. Note
that the changeover point is between tin and lead, though we have
already seen in the case of germanium's electrovalency, there are
idiosyncrasies.
(Click anywhere on diagram to
enlarge on a new web page) 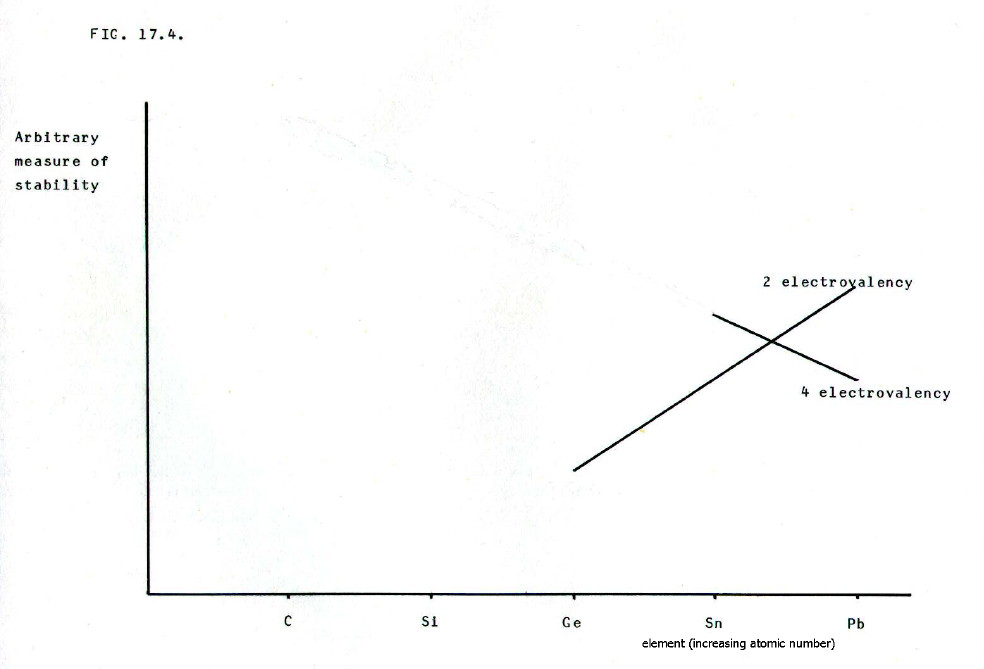
Bond energies and other relevant
energy changes can be used to check the predictions in sections i) to
iii). Redox potentials are also useful, especially when making
predictions about the reducing power of Sn2+ or
the oxidising power of Pb4+ ions in
aqueous solution.
iv) To summarise sections 17.3.1. and 17.3.2., group IV elements form both divalent and tetravalent compounds in which the bonding is mainly covalent. However, down the group it becomes increasingly ionic for the divalent state. Also, the divalent state changes from the less stable to the more stable state.
Note that valency should not be confused with oxidation state (section 11.3.5.). Oxidation state depends at least as much on the relative electronegativities of the bonded atoms/groups as it does on the valency or type of bond. Thus carbon shows every oxidation state from -IV to +IV in its tetravalent compounds with hydrogen and chlorine.
17.3.3. Higher valencies: From silicon downwards, the outer shell possesses d-orbitals which can be used to accept pairs of electrons for dative covalent bond formation. One importance of this has already been discussed in section 14.3.2.ii.
The availability of d-orbitals is also exhibited in the existence of complexes, such as: SiF62-, SnCl62-, GeCl62-, but PbCl42-. The ionic nature of the central species clearly increases down the group, as does the general stability of complexes. Silicon forms very few complexes, whereas lead forms many.
4+ ion complexes such as Sn(OH)62-, Pb(OH)64-, SnCl64-, and PbCl64- are probably the only clear example of the existence of 4+ ions, apart from their occurrence in fluoride lattices and perhaps in lead (IV) oxide.
17.3.4. Catenation: Carbon's particularly small size at the head of the group enables it to form particularly strong bonds with itself, and this is the basis of its ability to form almost indefinitely long chains of identical atoms (catenation). This is fundamental to organic chemistry.
The range of organic compounds is further increased by another relatively unique feature of carbon, its ability to form multiple bonds with itself and other elements. However, carbon does not fom quadruple bonds, so it always has at least one remaining electron involved in other bonds, and even in catenation.
Silicon has only one more shell than carbon but a far reduced ability to catenate. The maximaum chain length is around 6. Silicon's nuclei are further from the bonding electrons and therefore less strongly attracted to them and less stronly held together. However, silicon forms stronger bonds with the smaller oxygen atom, and can form long chains with the repeating -Si-O- structure. Such chains are found in silicates and silicones. Silicone greases and silicone rubbers are particularly important everyday compounds.
Silicon does not form multiple bonds.
17.4. SELECTED REACTIONS OF THE ELEMENTS
17.4.1. With oxygen: The strong covalent bonds in carbon and silicon mean that they react so slowly at room temperature as to be fairly inert. They are more reactive on strong heating, thus carbon is an important reducing agent in the blast furnace:
.......................................... 1500°C
Fe2O3(s)... +...
3C(s, coke)......  ......
2Fe(l)... +...
3CO(g)
......
2Fe(l)... +...
3CO(g)
In addition, carbon is used to reduce zinc oxide to zinc metal. It also burns in air/oxygen to give mixtures of carbon monoxide and dioxide. The proportion of carbon monoxide increases as the temperature increases and/or the supply of oxygen is limited.
The elements silicon to lead also form oxides when heated with oxygen. The reaction produces the dioxide except in the case of lead which produces the monoxide, illustrating the principles discussed in section 17.3.2. Lead (IV) oxide is usually prepared by the action of nitric(V) acid, an oxidising agent, on dilead(II)lead(IV) oxide (red lead):
Pb3O4(s)..+..4HNO3(aq)...  ...
PbO2(s).
+.
2Pb(NO3)2(aq). +.
2H2O(l)
...
PbO2(s).
+.
2Pb(NO3)2(aq). +.
2H2O(l)
17.4.2. With dilute acids: Only tin and lead are sufficiently electropositive to react with dilute acids:
M(s)... +...
2H+(aq)......  ......
M2+(aq)... +...
H2(g)
......
M2+(aq)... +...
H2(g)
This is predictable from the arguments in sections 17.3.1&2, and is another example of the effect down the group of the increasing number of shells. Lead does not react with dilute sulphuric(VI) acid because a thin protective layer of the insoluble sulphate forms on its surface.
The only acid to react with silicon is hydrogen fluoride. The reaction is similar to that of tin and lead with dilute acids, and SiF62- complex formation aids the reaction.
17.4.3. With concentrated oxidising acids: Even carbon reacts with hot concentrated sulphuric(VI) or nitric(V) acids:
C(s)... +...
2H2SO4(conc).....  .....
CO2(g)... +...
2H2O(l)... +...
2SO2(g)
.....
CO2(g)... +...
2H2O(l)... +...
2SO2(g)
C(s)... +...
4HNO3(conc).....  .....
CO2(g)... +...
2H2O(l)... +...
4NO2(g)
.....
CO2(g)... +...
2H2O(l)... +...
4NO2(g)
Germanium and tin react with concentrated nitric acid in the cold, and lead does not even require concentrated nitric(V) acid. This increasing ease (rate) of reaction reflects the increasing ease with which bonding in the element is broken, yet another result of the increasing number of shells down the group.
In addition, germanium and tin are oxidised to the +IV state, whereas lead is oxidised only as far as +II. Moreover, germanium and tin reduce the nitric(V) acid to NO, whereas lead reduces the nitric(V) acid only as far as NO2.
3Ge(s).. +..
4HNO3(conc)...  ...
3GeO2(s)..
+..
2H2O(l)..
+.. 4NO(g)
...
3GeO2(s)..
+..
2H2O(l)..
+.. 4NO(g)
3Sn(s).. +..
4HNO3(conc)...  ...
3SnO2(s)..
+..
2H2O(l)..
+.. 4NO(g)
...
3SnO2(s)..
+..
2H2O(l)..
+.. 4NO(g)
Pb(s).. +..
4HNO3(dil or conc)...  ...
Pb(NO3)2(aq).. +..
2H2O(l)..
+.. 2NO2(g)
...
Pb(NO3)2(aq).. +..
2H2O(l)..
+.. 2NO2(g)
There is little reaction between tin and concentrated sulphuric(VI) acid though it reats on heating. Lead similarly reacts with hot concentrated sulphuric(VI) acid, the heat in this case being needed to overcome the protective layer of sulphate:
M(s).. +..
2H2SO4(conc)..  ..
MSO4(s)..
+..
2H2O(l)..
+.. SO2(g)
..
MSO4(s)..
+..
2H2O(l)..
+.. SO2(g)
17.4.4. With alkali: All the group IV elements except carbon react with concentrated alkali. Tin and lead dissolve very slowly even when hot concentrated alkali is used. Even this reflects tin's and lead's extra shells and their increasing electropositivity:
Si(s).. +..
2-OH(aq)..
+.. H2O(l).....  .....
SiO32-(aq).. +..
2H2(g)
.....
SiO32-(aq).. +..
2H2(g)
Ge(s).. +..
2-OH(aq)..
+.. 4H2O(l).....  .....
Ge(OH)62-(aq).. +..
2H2(g)
.....
Ge(OH)62-(aq).. +..
2H2(g)
Pb(s).. +..
4-OH(aq)..
+.. 2H2O(l).....  .....
Pb(OH)64-(aq)
.....
Pb(OH)64-(aq)
If air is present, tin reacts like germanium, but in the absence of air it reacts like lead, another example of the gradual change in properties in this region of the periodic table.
17.5. COMPOUNDS: HYDRIDES, CHLORIDES AND OXIDES
Many of the important characteristics of carbon's and silicon's hydrides, chlorides, and oxides have already been discussed in chapter 14. Predictions about further changes down the group can be made by applying the principles used in that chapter to predict changes across a period. However, instead of increasing effective nuclear charge being the basis of predictions, down group IV predictions must be based on the increasing number of shells.
Some important changes in properties of group IV compounds are summarised in table 17.1. You should work out a basis for predicting the facts summarised the table so that you can learn the facts only as illustrations of your predictions.
| TABLE 17.1. Summary of properties of group IV hydrides, halides, and oxides, which illustrate predictable changes down the group. | |||
| Element | Properties
of MH4 (+ existence of chains) |
Properties of MCl2 | Properties of MCl4 |
| C | covalent,
almost unlimited chain length, stable m.p. - b.p. -191.34°C burns in air unaffected by alkali |
covalent very unstable |
covalent very stable m.p. -22.84°C b.p. 76.69°C not hydrolysed |
| Si | covalent,
Si-Si chain stable  6 6m.p. -185°C b.p. -112°C spontaneously burns in air violently hydrolysed by alkali |
covalent very unstable |
covalent stable m.p. -70°C b.p. 57°C very readily hydrolysed |
| Ge | covalent,
Ge-Ge chain stable  2 2not hydrolysed by h30% alkali |
covalent very unstable hydrolysed with oxidation |
covalent fairly stable m.p. -49°C b.p. 83°C reversibly hydrolysed |
| Sn | covalent,  elements at 150°C
elements at 150°Cm.p. -150°C b.p. -52°C not hydrolysed by  15%
alkali 15%
alkali |
partially
ionic stable m.p. 247°C b.p. 623°C reducing agent reversibly hydrolysed |
covalent fairly stable m.p. -33°C b.p. 113°C reversibly hydrolysed |
| Pb | covalent,  elements at 150°C
elements at 150°C |
ionic stable m.p. 498°C b.p. 954°C not hydrolysed |
covalent unstable  PbCl2
+ Cl2, yellow liquid at r.t. PbCl2
+ Cl2, yellow liquid at r.t.m.p. -33°C b.p. 113°C rapidly hydrolysed |
| TABLE 17.1. continued | ||
| Element | Properties of MO | Properties of MO2 |
| C | covalent
discrete molecules stable m.p. -205°C b.p. -191°C reducing neutral |
covalent,
discrete molecules stable m.p. -56°C b.p. -78°C acidic |
| Si | covalent
discrete molecules unstable reducing exists only at high temps. |
covalent,
macromolecular stable m.p. 1610 1713°C, depending on form b.p. 2230°C amphoteric |
| Ge | covalent,
macromolecular unstable sublimes at 710°C reducing amphoteric |
covalent,
macromolecular stable m.p. 1116°C amphoteric |
| Sn | partially
ionic fairly unstable decomposes at 1080°C reducing amphoteric |
covalent
macromolecular stable m.p. 1127°C b.p. 1800°C amphoteric |
| Pb | ionic stable m.p. 886°C b.p. 2358°C amphoteric |
ionic
with strong covalent character decomposes  PbO + O2
on heating PbO + O2
on heatingdecomposes at 300°C oxidising amphoteric |
notes i) The word stable has been used. When it is used, a key question is: "Stable with respect to what?". Note that a compound which is thermally stable with respect to its elements may not be stable to oxidation by air or to hydrolysis by water/alkali.
notes ii) The hydrolysis of carbon and silicon compounds has been discussed in chapter 14.
Hydrolysis of tin and lead compounds is slightly more complicated. Thus, dissolving tin(II) chloride in water gives a cloudy solution owing to precipitation of basic chlorides such as:
2SnCl2(s). +.
2H2O(l)..  ..Sn(OH)2.SnCl2(aq). +.
2H+(aq). +.
2Cl-(aq)
..Sn(OH)2.SnCl2(aq). +.
2H+(aq). +.
2Cl-(aq)
It can be predicted from this that hydrolysis is limited in hydrochloric acid solution. Acidified solutions containing SnCl3- and SnCl42- ions are generally used in the laboratory. Moreover tin is kept in the bottle to prevent oxidation to tin(IV) chloride. Lead(II) chloride which is sparingly soluble in cold water, but readily soluble in hot water also undergoes partial, reversible hydrolysis.
The tetrachlorides are rapidly hydrolysed:
MCl4(l)..... +..... 2H2O(l)........  ........
MO2(s)..... +..... 4HCl(g)
........
MO2(s)..... +..... 4HCl(g)
The aqueous solution of tin(IV) chloride also contains Sn(H2O)64+ ions. With concentrated hydrochloric acid, both form soluble complexes, SnCl62- and PbCl62-.
notes iii) The acid base reactions of carbon and silicon oxides have already been discussed in chapter 14.
The oxides of tin and lead are amphoteric. Thus the monoxides form salts with acids:
MO(s)..... +..... 2H+(aq)........  ........
M2+(aq)..... +..... H2O(l)
........
M2+(aq)..... +..... H2O(l)
Complex formation with the anion of the acid may be important in some cases e.g. with concentrated hydrochloric acid. The monoxides also form stannates(II) and plumbates(II) with alkalis:
MO(s)..... +..... 2-OH(aq)..... +..... H2O(l)........  ........
M(OH)42-(aq)
........
M(OH)42-(aq)
This anion occurs in a series of equilibrium reactions similar to that described for Al3+ in section 13.9.4.:
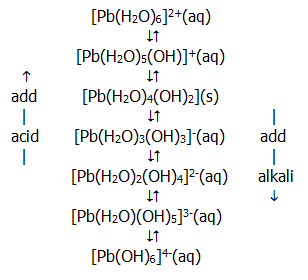
The dioxides also form salts with acids:
SnO2(s).... +.... 2H2SO4(conc)....  ....
Sn(SO4)2(aq)....
+.... 2H2O(l)
....
Sn(SO4)2(aq)....
+.... 2H2O(l)
................................................ ice cold
PbO2(s)....
+.... 6HCl(conc).....  .....
2H+(aq)....
+.... PbCl42-(aq)....
+.... 2H2O(l)
.....
2H+(aq)....
+.... PbCl42-(aq)....
+.... 2H2O(l)
Note that at higher temperatures there is significant oxidation of the chloride ions to chlorine by lead(IV) oxide!
The dioxides also dissolve in concentrated alkalis:
MO2(s)....
+.... 2-OH(aq)....
+.... 2H2O(l).......  ....... M(OH)62-(aq)
....... M(OH)62-(aq)
17.6. QUESTIONS
1) Write the formulae of methane and its chloroderivatives which show a range of carbon oxidation states from -IV to +IV.
2) What is the difference between valency and oxidation state? Illustrate your answer by considering the fact that the number of bonds formed by carbon in methane is greater than the number of bonds formed by carbon in carbon monoxide, and yet the oxidation state of carbon in methane is less than its oxidation state in carbon monoxide.
3) Explain why you are not surprised that PbI4 does not exist.
4) Using redox potentials from a data book, predict and write balanced equations for any redox reactions that may occur between any pairs of the following ions: Pb4+, Pb2+, Sn4+, Sn2+, I-, Fe2+, Fe3+.
5) Explain how SnF4 and PbF4 are amongst the only lattices in which M4+ ions exist. Explain how the 4+ ions can exist in aqueous solution.
6) Describe in more detail the basis of carbon's ability, but nitrogen's inability, to catenate. Also describe (and explain the basis for) the difference between multiple bond formation by carbon and by nitrogen.
7) How do the reactions of group IV elements with oxygen illustrate the principles discussed in section 17.3.2? How could the rather idiosyncratic stability of carbon monoxide be predicted?
8) Why must sulphuric(VI) and nitric(V) acids be hot and concentrated in order to react with carbon? How do you explain the lack of silicon's reactivity with these acids?
9) Write balanced equations, showing changes in oxidation state, for the reactions of germanium, tin and lead with nitric(V) acid? How could the differences in the reactions have been predicted?
10) Would you predict that germanium reduces concentrated sulphuric acid? If so, under what conditions? Explain your reasoning.
11) Addition of dilute hydrochloric acid to a solution of lead(II) nitrate produces a white precipitate. Further addition of concentrated nitric acid causes the precipitate to redissolve. Describe the chemical changes occurring at each stage.
12) In some respects carbon behaves more like lead than silicon. List examples and describe the chemical basis of carbon's anomalous behaviour in each case.
13) How do the reactions of group IV elements with alkali reflect the increasing number of shells down the group? Describe the basis of carbon's anomalous behaviour.
14) Relate the changing structures down group IV of the hydrides, chlorides and oxides, to the increasing number of shells in the group IV elements.
Then relate the changing physical and chemical properties of the compounds to their changing structures (and hence changing number of shells in the group IV elements).
15) Explain why the group IV dioxides are generally more acidic than the correspoding monoxides.
Unless otherwise stated, all materials in this web version of chapter 17 are © 2007 Adrian Faiers MA (Oxon) MCIPR

What 's the connection between a dozen eggs and
a garden mole?

Answer: Not a lot, really, but see Chapter 1