Recommended by:
Top
20 UK science resources,
The Tutor Website
Recommended by:

Rated:

2010
Listed on the science,
engineering
and technology section of 
'providing you with access
to the very best Web resources for education and research, evaluated
and selected by a network of subject specialists.'
(Please note that intute closed in July 2011)
Section 2: Inorganic
CHAPTER 18: THE
FIRST ROW OF d-BLOCK ELEMENTS
(including transition elements)
NB This chapter has now been
updated to improve browser compatibility.
Please
use the 'send email' link at the top right hand corner of this page to
report any problems.
18.1. INTRODUCTION
18.1.1. Distinctions: Within the d-block of the periodic table there is a group of elements which show characteristic properties. They are called transition elements. Not all the elements in the d-block are transition elements; not all show the characteristic reactions to such an extent.
All the d-block elements have partially filled or just-filled penultimate (inner) d-orbitals in the neutral atom, but no outer p-electrons.
In contrast, transition elements have been defined in two ways:
i) Elements with partially filled (inner) d-orbitals in the neutral atom. In the first row this would include all the elements from scandium to nickel.
ii) Elements with partially filled (inner) d-orbitals in at least one of their ions. In the first row this would include all the elements from titanium to copper.
The second definition is better for two, connected, reasons:
First, scandium shows few of the characteristic properties of those elements which are included in both definitions, whereas copper shows them all.
Second, the characteristic properties are mainly properties of compounds which depend on the partially filled d-orbitals in those compounds.
18.1.2. The characteristic properties of transition elements are:
i) They are all hard, not particularly electropositive metals, with similar (relatively small) atomic radii.
ii) They form a wide range of complexes
ii) They show variable oxidation state
iv) They form coloured compounds
v) They show magnetic properties.
vi) They show considerable catalytic activity
vii) They readily form alloys with each other.
Note that none of these properties is unique to transition metals. However, there is no other group of elements which shows them all. Moreover, transition elements show each of the properties to a considerable extent.
Note also, that many of the properties are interrelated. In particular, the involvement of partially filled d-orbitals is an underlying theme, though sometimes it is overemphasised.
In this chapter, we shall look mainly at these characteristic properties, but note also that there are changes across the period due to changes in effective nuclear charge.
18.1.3. Brief note on effective nuclear charge: Across the period (d-block) the ratio of protons to electron shells increases. This leads to an increase in effective nuclear charge. Moreover, d-orbitals are not particularly good at screening (section 13.2.).
However, there is a complication. Repulsion occurs within the d-subshell as further electrons are added. The result is that effective nuclear charge does not increase as rapidly as might be predicted.
This is demonstrated in the variation of first, second and third ionisation energies which hardly increase across the series. Also, atomic radii decrease to chromium, but remain virtually identical for the rest of the series, until there is actually a slight increase to copper and zinc.
In addition, idiosyncrasies occur at Cr/Mn and Cu/Zn. The even distribution of charge in each of these pairs leads to the electrons being held more tightly than might be expected. The even charge distribution is a function of the symmetrical distribution of electrons in half-filled (Cr/Mn) or completely filled (Cu/Zn) d-orbitals (c.f. section 13.6.2.).
Note that chromium would not have half-filled d-orbitals, and copper would not have completely-filled d-orbitals, if it were not for the greater attraction of the nuclei for even charge distribution (c.f. section 2.4.2. and FIG. 2.6.).
The
Cr/Mn and Cu/Zn idiosyncrasies are clearly seen in the variation of
melting point and boiling point in FIG 18.1.
(Click anywhere on diagram to
enlarge on a new web page) 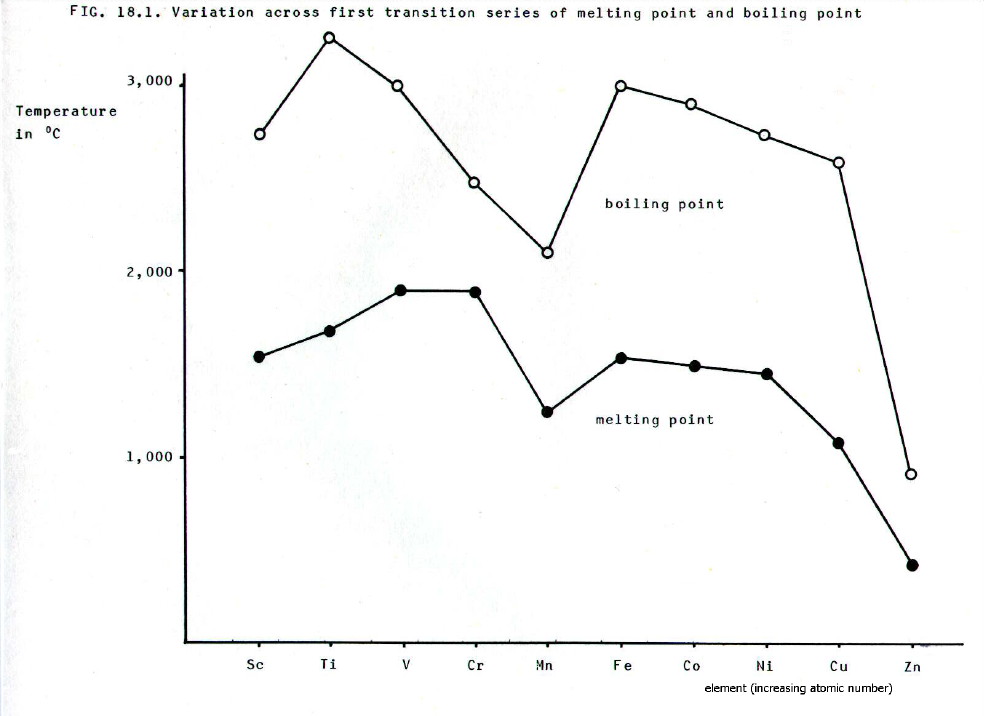
Due to the increased attraction
of the nuclei for the evenly distributed electrons in the half-filled
d- and s-orbitals of chromium, the outer electrons are less involved in
the metallic bonding orbital. The metallic bonding is therefore weaker
than expected and chromium's melting and boiling points are lower than
expected. At manganese, which has an extra proton to compound the
effect, there is actually a drop in melting and boiling points.
Moreover, the electrons in completely filled d-orbitals are even less available for metallic bond formation due to the favourable electromagnetic interaction of spin-pairing. This, combined with the effect of the even charge distribution, leads to the drop in m.p. and b.p. between nickel and copper, and the further drop to zinc. The pairing and even charge distribution effects also account for the slight increase in atomic radius shown at copper and zinc.
18.2. COMPLEX FORMATION
18.2.1. A complex comprises a central positive ion (or a neutral atom in the case of Ni(CO)4) surrounded by ligands which are negative ions or neutral molecules (or, rarely, positive ions) with at least one pair of electrons involved in dative covalent bond formation with the central ion/atom.
E.g. hexaaquachromium (III):
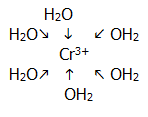
18.2.2. As befits their title the naming of complex ions is complicated. There is a set of rules. First, in compounds containing complex ions, the cation is named before the anion. Then, in the complex itself:
i) The ligands are named before the central ion/(atom).
ii) Ligands are named in the order: anions, then neutral molecules, then cations.
iii) If there is more than one ligand falling into one of these categories, they are named in alphabetical order.
iv) The names of anionic ligands end in -o (e.g. cyano, chloro, hydroxo etc.), those of neutral ligands usually stay unaltered except: water = aqua, ammonia = ammine, and carbon monoxide = carbonyl, and those of anions end in -ium (e.g. NH2-NH3+ = hydrazinium).
v) If there is more than one ligand of a species which already contains a prefix like di- or tri- in its name (e.g. ethane-1,2-diamine) then, to avoid confusion, that type of prefix is not used again in the naming of the complex, but replaced by bis-, tris-, tetrakis- etc. In addition, the ligand name is placed in brackets.
vi) The name of metals forming complex anions is terminated with -ate, whereas the usual name is kept when neutral or cationic complexes are formed.
vii) The oxidation state of the central ion/(atom) is indicated by roman numerals in brackets immediately after its name.
A few illustrative examples:
| Formula | Name |
| [Mn(H2O)6]SO4 | hexaaquamanganese(II) sulphate |
| [Ni(CO)4] | tetracarbonylnickel(0) |
| [Cu(NH3)4(H2O)2]SO4 | tetraamminediaquacopper(II) sulphate |
| [Co(NH3)5(NO2)]Cl2 | nitropentaamminecobalt(III) chloride |
| K3[Fe(CN)6] | potassium hexacynoferrate(III) |
| [Co(NH2CH2CH2NH2)3]Cl3 | tris(ethane-1,2-diammine)cobalt(III) chloride |
18.2.3. Like other characteristic properties of transition elements, complex formation is not unique to these elements. Even group I metal ions are hydrated in aqueous solution. However, transition metals are particularly good at it for two reasons:
i) Their ions are small and tend to be highly charged i.e. they have high surface charge densities and strongly attract ligands.
ii) Amongst other orbitals, they have available inner d-orbitals to accept the ligand electrons.
Note that the high surface charge density is the key factor. Note also that the role of the partially filled d-orbitals in complex formation is often exaggerated. The strong attraction for ligands allows more orbitals than just the partially filled d-orbitals to accommodate the ligand electrons. Even d-orbitals in the next energy level are used in some cases.
18.2.4. i) The complexes of cobalt 2+ and 3+ provide a useful study of the orbitals used in complex formation (study questions 18.3. and 18.7.).
The cobalt atom in the ground state has the outer electron configuration:
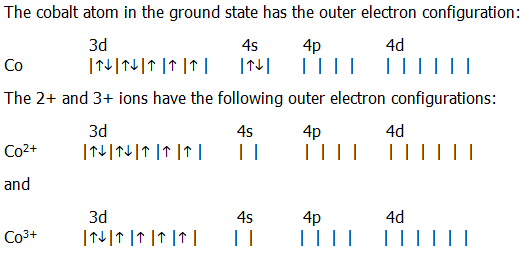
With weak ligands, such as F- (section 18.4.3.), both ions form octahedral complexes in which the ligand electrons are accommodated in sp3d2 hybrid orbitals. In other words, the partially filled inner d-orbitals are not used. This type of complex is known as an outer d-orbital complex.
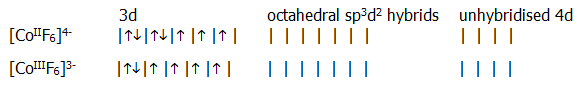
With strong ligands, such as -CN ions (section 18.4.3.), spin-pairing of the inner d-electrons, occurs and both ions form octahedral complexes in which the ligand electrons are accommodated in d2sp3 hybrids. In other words the partially filled d-orbitals are used, and this type of complex is known as an inner d-orbital complex.
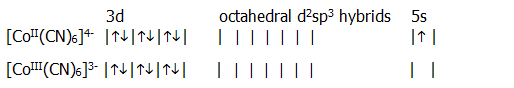
ii) Spin-pairing has two interesting effects:
First, it reduces paramagnetism which is associated with unpaired spinning electrons. In a similar war to any circular current, such spinning electrons have associated magnetic fields. Clearly, spin-pairing generally reduces paramagnetism (section 18.5.2.).
Second, it promotes the "third" electron in Co2+ into a 5s orbital, making it easier to lose, and stabilising the 3+ ion with respect to the 2+ ion. This is a good example of just how variable the valency of transition metals can be (section 18.3). In general, spin-pairing often stabilises or destabilises one oxidation state with respect to another.
18.2.5. Shapes: The complexes of cobalt desribed above are octahedral in shape (section 4.9.). Those with four ligands tend to be tetrahedral, and those with two ligands linear. However, some complexes with four ligands are described as square planar, although sometimes this is because they have two water ligands as well as the ligands which show the square planar arrangement. Such complexes are really octahedral.
Apart from applying electron repulsion theory to predict shapes, the concept of orbital hybridisation is also a useful tool (section 4.8.).
Note also, that especially those complexes with more than one type of ligand are capable of showing isomerism.
18.3. OXIDATION STATE
18.3.1. Two attributes of oxidation state change across the first row of d-block elements:
i) Maximum oxidation state
ii) The relative stabilities of the (variable) oxidation states
A key factor is the similar attraction of the nucleus for electrons in both 4s and 3d orbitals. The relative strength of attraction changes as nuclear charge increases, and as d-electrons interact with s-electrons.
In addition, the attraction of the nucleus for electrons in different d-orbitals is equal, though this may change when ligands bind to transition elements and their ions (section 18.4.).
All these relative strengths of attraction are reflected in the energy levels of the orbitals (section 2.4.3. and FIG. 2.6.).
18.3.2. Maximum oxidation state first of all increases as the total number of available 4s plus 3d electrons increases. It reaches a maximum at VII with manganese, but then decreases as the increasing effective nuclear charge reduces the ability to lose, or lose control of, the electrons. As a rough guide, maximum oxidation state = the total number of 4s + unpaired 3d electrons.
Compounds containing transition elements in their highest oxidation states are often powerful oxidising agents, the best known being the manganate(VII) ion, MnO4-, and the dichromate(VI) ion, Cr2O72-. Note that the orange dichromate(VI) ion exists only in acid solution. In alkaline conditions it breaks down into the yellow chromate(VI) ion:
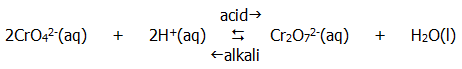
Purple manganate(VII) ions are most commonly reduced to colourles Mn2+ ions in acidic solution, and most commonly to brown solid manganese(IV) oxide in alkaline solution. Dichromate(VI) ions are usually reduced to green hydrated Cr3+ ions. Hydrated Cr2+ ions are sky blue in colour.
18.3.3. The relative stabilities of the various oxidation states are well illustrated by the changes in stability of the 3+ with respect to the 2+ ion in aqueous solution. As effective nuclear charge increases across the series, we would predict that it will be increasingly difficult for M2+ to lose a third electron and form M3+. This is roughly the case, a fact that is reflected by the redox potentials for the 3+/2+ equilibria set out in table 18.1:
| TABLE 18.1. | |
| Element | M3+/M2+ redox potential in V |
| Sc | Forms only the 3+ ion |
| Ti | -0.37 |
| V | -0.26 |
| Cr | -0.41 |
| Mn | +1.51 |
| Fe | +0.77 |
| Co | +1.82 (in molar nitric(V) acid) |
| Ni | Ni3+ highly oxidising |
| Cu | 3+ ion insignificant |
| Zn | Forms only the 2+ ion |
Just looking at the trend in values indicates that chromium has a slightly more negative value than expected, manganese has a more positive value than expected, and iron has a less positive value than expected. Though, note that it is easier to decide which values are idiosyncratic in the light of predictions.
The more negative the redox potential, the more favoured is the 3+ ion. The more positive the redox potential, the more favoured is the 2+ ion.
Applying this to each of the idiosyncrasies referred to above, it seems that:
i) The Cr3+ ion is more favoured than expected. This is because loss of the "third" electron makes room for accommodation of electrons from six water ligands in d2sp3 hybrids. In other words this allows the ligand electrons to use orbitals closer to the central ion's nucleus and the forces of attraction are therefore greater.
ii) The Mn2+ ion is more favoured than expected. This is because it has the even charge distribution associated with half-filled d-orbitals. Formation of the 3+ ion destroys the symmetry.
iii) The Fe3+ ion is more favoured than expected. This is because its formation from the 2+ ion produces half-filled d-orbitals, whereas the 2+ ion has an uneven distribution of charge in the d-orbitals.
18.3.4. Summary of possible oxidation states:
| TABLE 18.2. The main oxidation states of the elements in the first row of the d-block. Commom oxidation numbers are underlined. Less stable oxidation states are shown in brackets. The oxidation states in the main oxides and chlorides are also shown. | ||||||||||
| Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | |
| Main oxn. states | 7 | |||||||||
| 6 | (6) | (6) | ||||||||
| 5 | (5) | (5) | (5) | 5 | ||||||
| 4 | 4 | (4) | 4 | (4) | (4) | (4) | ||||
| 3 | 3 | 3 | 3 | (3) | 3 | 3 | (3) | (3) | ||
| (2) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | ||
| (1) | (1) | (1) | (1) | (1) | (1) | (1) | 1 | |||
| Main oxides | III | IV | V | VI | VII | III | III | II | II | II |
| III | III | III | IV | II | II | I | ||||
| II | ||||||||||
| Main chlorides | III | IV | III | III | III | III | II | II | II | II |
| III | II | II | II | I | ||||||
18.4. COLOURED COMPOUNDS
18.4.1. A clearer property of partially filled d-orbitals is colour. In discussing colour, the concept of energy is useful for linking electron movements against electrostatic forces with the colour of light absorbed.
The electrons in d-orbitals of transition elements are all attracted with the same electrostatic force; they have equal energies. However, when ligands bind to an atom or ion, there are two effects:
First, the ligand electrons repel electrons in the d-orbitals, raising their energy.
Second, they repel some of the orbitals more than others because they are closer to them. This has the effect of splitting the d-orbitals into two energy levels.
Electrons can be promoted from the lower d-level to the higher d-level by absorbing light with an energy (and corresponding colour) equal to the energy difference between the split d-levels. The observed colour of the transition metal compound is the light which is not absorbed and which is transmitted or reflected. When the electrons return to the lower level, energy is released as heat rather than light.
The colours of various transition metal ions have been given above, but perhaps the most familiar is the blue colour of [Cu(H2O)6]2+. The ion is described as hydrated, as are salts containing the hydrated ion. If the water ligands are removed, for example by heating the hydrated solid, the blue colour *disappears in the resulting, so-called, anhydrous compound. The water molecules surrounding the copper (II) ion in crystals of, e.g., hydrated copper (II) sulfate are known as water of crystallisation. They remain in the solid when it is crystallised from aqueous solution and it is these which are driven off when the hydrated crystals are heated to form the anhydrous salt.
It is clearly possible to apply the understanding in Chapter 1, section 1.4.10 to calculate the formula of a hydrated salt given percentage composition, mass composition or based on experimental results.
*Moreover, when four of the water ligands are replaced by ammonia ligands, the colour changes to a much deeper blue (see appendix i and question 18.3.).
18.4.2. More on the splitting: The d-orbitals fall into two groups: Two orbitals (eg group) which occupy space in the atom concentrated on the x,y, and z axes, and
Three orbitals (t2g group) which are concentrated between the x, y, and z axes.
In octahedral complexes, the ligands lie on the x, y, and z axes. The ligand bonding pairs therefore repel electrons in the eg orbitals more than those in the t2g orbitals. In energy terms, the eg orbitals are raised to a higher energy than the t2g orbitals.
In tetrahedral complexes, the ligands lie inbetween the x, y, and z axes. Thus electrons in the t2g orbitals are repelled more, and the t2g orbitals are raised to a higher energy than the eg orbitals.
18.4.3. The amount of splitting depends on the strength of the ligand and the electrostatic field it creates (scetion 18.2.4.i.). The ligands below are shown in order of decreasing "crystal field effect":
-CN > NO2- > NH3 (and amines) > H2O > F- > -OH > Cl- > Br- > I-
18.5. MAGNETIC PROPERTIES
18.5.1. Diamagnetism: All substances are diamagnetic. They are very weakly repelled by conventional magnets owing to the magnetic fields associated with the orbital motion of their electrons. Diamagnetic effects are so weak that they are completely masked when a substance is either paramagnetic or ferromagnetic.
18.5.2. Paramagnetism: As mentioned in section 18.2.4.ii., paramagnetism is due to the magnetic field associated with an unpaired electron, which can be compared with the magnetic field created by a circular current. Paramagnetic substances are attracted by conventional magnets; the magnetic fields of the individual atoms become alligned with the external field. The greater the number of unpaired electrons, the stronger the attraction.
Both transition metal atoms and their ions may have unpaired electrons, though the number of unpaired electrons in an ion is greatly affected by ligands (sections 18.2.4.ii. and 18.4.3.).
18.5.3. Ferromagnetism: Ferromagnetism occurs in iron, cobalt, and nickel (plus certain alloys). The ionic spacing in these elements is such that inter-atomic forces known as exchange forces operate between them and adjacent paramagnetic atoms organise into groups known as domains.
These domains interact more strongly with external magnetic fields than individual atoms, and ferromagnetic substances are strongly attracted by conventional magnets.
Moreover, not only do the domains become aligned with the external field, but when it is removed, they remain so. The rate at which they return to their random positions depends on the type of metal, temperature etc., but in this way, ferromagnetic substances produce what we have, in this section, been calling conventional magnets, i.e. permanent magnets.
18.6. CATALYSIS
18.6.1. Two properties of transition elements are particularly important in their action as catalysts:
i) Their ability to reversibly adsorb certain substances, most important in heterogeneous catalysis (sections 9.10.3b. and 9.10.5b.).
ii) Their readily interconvertible oxidation states, most important in homogeneous catalysis (sections 9.10.3a. and 9.10.5a.).
An example of each type involving a transition element, is given in chapter 9. There are many industrial reactions which involve transition metal catalysts. Some are listed in table 18.2.
| TABLE 18.3. | ||
| Catalyst | Industrial process catalysed | Other conditions |
| TiCl3/Al2(C2H5)n | polymerisation of ethene | vary (section 20.12.5.) |
| V2O5 | manufacture of
sulphuric(VI) acid 2SO2 + O2  2SO3)
2SO3) |
450 °C (section 19.3.3.ii.) |
| Fe2O3/Fe | Manufacture of
ammonia (N2 + 3H2  2NH3)
2NH3) |
550 °C, 200
atmos. (section 19.3.2.) |
| Ni | Hardening of vegetable oils | 150 °C (section 25.3.1.) |
Transition metals also play a role in biological catalysis. Transition metal ions are found at the active site of some enzymes. In addition, iron is an important component of haemoglobin, which carries oxygen in blood. Magnesium (not a transition element) is an important component of chlorophyll, which couples the absorption of sunlight with synthetic reactions in plants.
18.7. ALLOYS
The main characteristic of transition elements which makes them good at forming alloys with each other, is their similar atomic radii. This allows ions of one metal to replace those of another in the crystal lattice, without causing undue distortion.
However, there is often some distortion of the lattice, and this may be important. Thus copper is soft, but introducing zinc atoms into the lattice makes the resulting alloy, brass, harder. The zinc atoms interrupt the orderly arrangement of ions in the lattice and prevent them from sliding over each other.
Alloys of copper with tin (bronze) have greater tensile strength than copper, itself, and can be more readily cast in moulds.
In general, metals are alloyed to give them more useful properties. Thus, up to 2% carbon is added to iron (note that pig-iron is brittle because it contains around 5% carbon plus silicon, sulphur and phosphorus impurities) producing the much stronger alloy, steel. Stainless steel contains 12-15% chromium to make it resistant to corrosion. Chrome-tungsten steel contains 4% chromium and 15% tungsten making it very hard, even when red hot. It is particularly useful for drill bits etc. Silcon steel contains around 5% silicon and cuts down electrical waste when used for transformer plates. (See also section 19.1.7.)
18.8. QUESTIONS
1) Write out electron-in-box structures like those used in section 18.2.4. to show the 3d and 4s electron configuations of the elements from scandium to zinc (N.B. section 18.1.3.)
2) Write balanced half equations for the reduction of the manganate(VII) ion in acid and alkaline conditions, and for the reduction of dichromate ions. By looking up redox potentials in a data book, predict some interesting examples of these ions acting as oxidising agents, and write balanced equations for them (two examples for each of the three cases).
3) When aqueous sodium hydroxide is added slowly to a pale blue solution of copper(II) sulphate, a pale blue precipitate of [Cu(H2O)4(OH)2] is produced. The precipitate remains when further sodium hydroxide is added. When ammonia solution is added slowly to a pale blue solution of copper(II) sulphate a pale blue precipitate of [Cu(H2O)4(OH)2] is again produced. However, the precipitate redissolves as more ammonia solution is added, producing a deep blue solution. Describe the changes, and also the basis of the different behaviour with sodium hydroxide and ammonia solutions.
4) Write formulae for the oxides and chlorides referred to in table 18.2. Predict the acid/base properties of the oxides, giving your reasons, and give equations to illustrate reactions with acid and/or alkali.
5) Co3+ ions can liberate oxygen from water and yet when aqueous KCN or aqueous ammonia is added to solutions containing Co2+ ions, air is found to oxidise the Co2+ ions to Co3+ ions. Explain this apparently contradictory behaviour.
6) Ammonium vanadate(V), NH4VO3 is dissolved in alkali. When sulphuric acid is added it produces a yellow solution. When the solution is shaken with granulated zinc or zinc amalgam, the solution turns through green to blue, to green again, and finally to violet. Explain the colour changes and write equations for all the chemical reactions given the following information:
VO2+ ions are yellow
VO2+ ions are blue
V3+(aq) ions are green
V2+(aq) ions are violet
7) When hexacynoferrate(III) ions are added to solutions containing aqueous iron(II) ions, a blue precipitate (Turnbull's blue) is produced. When hexacyanoferrate(II) ions are added to solutions containing aqueous iron(III) ions, an apparently darker blue precipitate (Prussian blue) is formed. Both precipitates contain [FeIIFeIII(CN)6]- ions and seem to be the same compound. Addition of Hexacyanoferrate(III) ions to solutions containing aqueous iron(III) ions produces a brown solution and addition of hexacynoferrate(II) to solutions containing aqueous iron(II) solutions produces a white precipitate. Tabulate all the observations. What changes do you think occur in these latter two cases, and suggest reasons why Prussian blue appears deeper in colour than Turnbull's blue?
Unless otherwise stated, all materials in this web version of chapter 18 are © 2007 Adrian Faiers MA (Oxon) MCIPR

What 's the connection between a dozen eggs and
a garden mole?

Answer: Not a lot, really, but see Chapter 1