Recommended by:
Top
20 UK science resources,
The Tutor Website
Recommended by:

Rated:

2010
Listed on the science,
engineering
and technology section of 
'providing you with access
to the very best Web resources for education and research, evaluated
and selected by a network of subject specialists.'
(Please note that intute closed in July 2011)
Section 2: Inorganic
CHAPTER 19:
CHEMISTRY VS.MONEY
(some inorganic industrial processes)
NB This chapter has now been
updated to improve browser compatibility.
Please
use the 'send email' link at the top right hand corner of this page to
report any problems.
19.1. EXTRACTION OF METALS
19.1.1. The extraction of metals is essentially a process of reduction:
.....................Mn+.... +....
ne-....... ....... M
....... M
The method used depends on how readily the reduction occurs. This in turn can be predicted from the position of the metal in the periodic table (sections 4.3. and 13.6. etc.) and is reflected by the position of the element in the electrochemical series (section 11.6.). Part of the electrochemical series is shown below:

19.1.2. Potassium to zinc: Electrolysis is used to extract these, the most electropositive elements. Reduction occurs at the cathode. The electrolyte is usually the chloride, which is fused. Aqueous solutions would contain hydrogen ions and these would be discharged in preference to the metal ions.
Often electrolytic extraction of metals is confined to geographical areas where cheap electricity is available, such as hyro-electric power in areas of Canada and Scandinavia. However, the cost of transporting the ore must be weighed against this consideration. Even political factors are involved. Wealthy industrialised nations often have protective policies which prevent poor developing countries from processing their own ores.
Two commonly studied examples of the method are the extraction of sodium and the extraction of aluminium.
19.1.3. Sodium: i) The ore is naturally occuring rock salt (NaCl)
........................ii) Calcium chloride is added to reduce the m.p. of the electrolyte from 800°C to 600°C. This not only reduces the heating bill, it prevents sodium (b.p. 800°C) from vapourising (sodium vapour would ignite in air), and reduces corrosion of the cell materials by chlorine and the liquid sodium.
........................iii) The reactions at the electrodes are:
anode:.............. Cl-..... -.....
e-.....  .....
..... 
.....................................2 .....
.....  .....
Cl2
.....
Cl2
cathode:.........Na+.....
+..... e-.....  ..... Na
..... Na
(Click
anywhere on the diagram to enlarge it on a new web page) 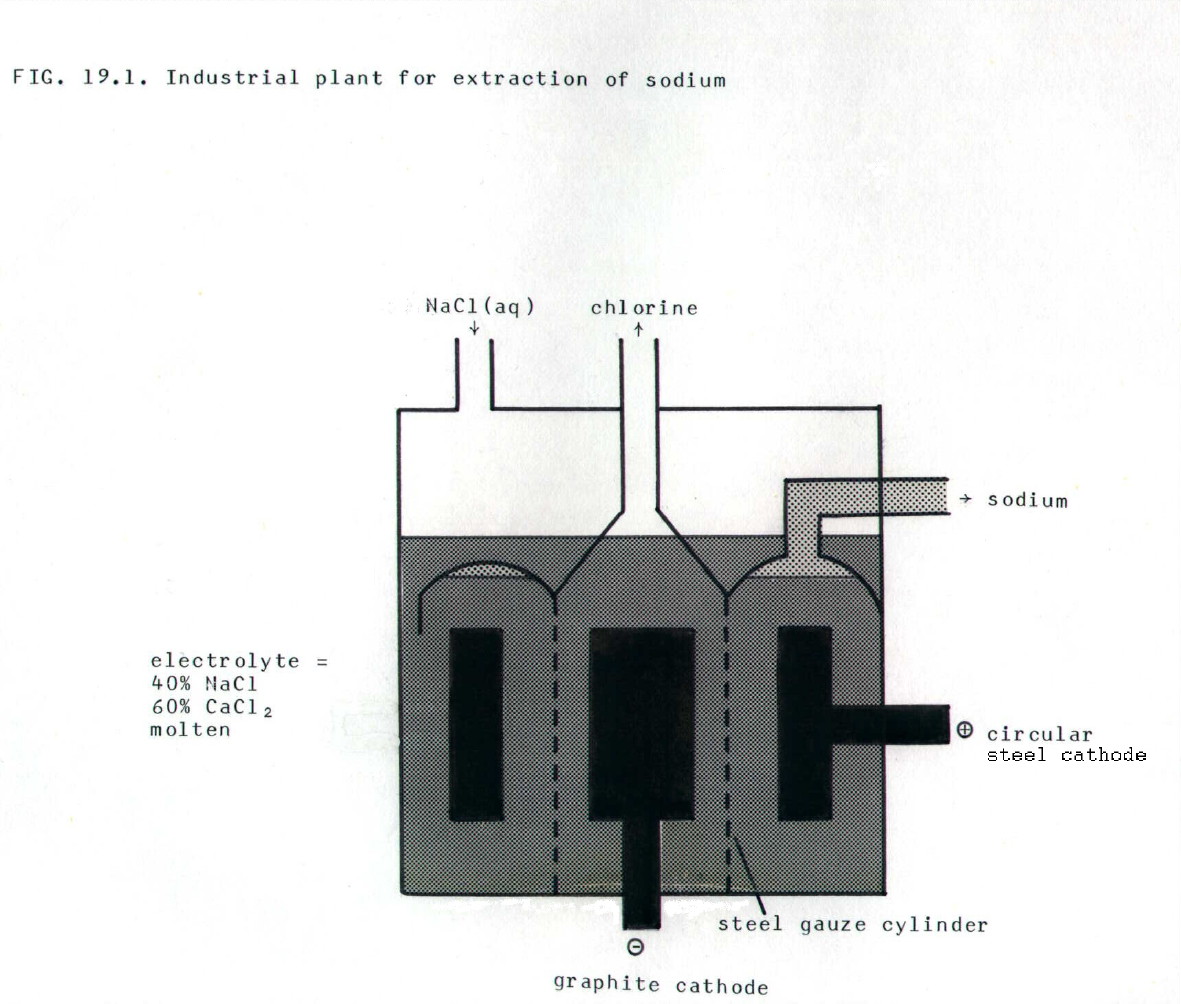
19.1.4. Aluminium: i) The ore is bauxite, hydrated
aluminium(III) oxide, Al2O3.xH2O,
with impurities of iron(III) oxide and silicon(IV) oxide. Purification
of the ore takes advantage of the amphoteric nature of the oxide:
Powdered bauxite is heated under pressure with concentrated aqueous sodium hydroxide. This dissolves the amphoteric aluminium(III) oxide and some of the acidic silicon(IV) oxide. It does not dissolve the basic iron(III) oxide which is filtered out, together with other insoluble impurities:
Al2O3(s)..... +.....
2OH-(aq)..... +..... 3H2O(l).......  .......
2[Al(OH)4]-(aq)
.......
2[Al(OH)4]-(aq)
SiO2(s)..... +..... 2OH-(aq).......  .......
SiO32-(aq)..... +..... H2O(l)
.......
SiO32-(aq)..... +..... H2O(l)
The solution is cooled and the pH is then lowered either by dilution (cheap) or by bubbling carbon dioxide through it. This precipitates the amphoteric aluminium(III) hydroxide, but the acidic silicon(IV) oxide stays in solution. When the dilution method is used, the solution is also "seeded" with a little freshly prepared aluminium(III) oxide:
[Al(OH)4]-(aq).......  .......
Al(OH)3(s)..... +..... -OH(aq)
.......
Al(OH)3(s)..... +..... -OH(aq)
Not all the hydration has been shown in these equations (section 13.9.4.)
Pure aluminium(III) oxide is obtained from the hydroxide by heating it in kilns:
2Al(OH)3(s).......  .......
Al2O3(s)..... +..... 3H2O(l)
.......
Al2O3(s)..... +..... 3H2O(l)
For reasons of economy, the sodium hydroxide solution is re-cycled. Morover, purifying the ore at this stage is much cheaper than trying to purify an electropositive metal. Simple chemical methods such as those used to purify iron metal would be impossible.
............................. ii) Up to 5% cryolite (Na3AlF6) together with some calcium chloride is added to the electrolyte to lower its melting point and therefore heating costs. Cryolite is a scarce mineral and most is now made synthetiically. It is not electrolysed.
............................ iii) A small amount of aluminium(III) fluoride is added to reduce the solubility of aluminium in the molten electrolyte. Molten aluminium then sinks to the bottom and is run off.
........................... iv) The electrolyte is continually replenished with aluminium(III) oxide.
........................... v) The carbon anodes must be regularly replaced because oxygen released at the anodes attacks them to form carbon dioxide and some carbon monoxide.
.......................... vi) The heating effect of the current keeps the electrolyte molten.
......................... vii) The electrode reactions are:
anode:..... 4AlO33-..... -.....
12e-.....  .....
2Al2O3..... +.....
3O2
.....
2Al2O3..... +.....
3O2
cathode:........ Al3+..... +.....
3e-.....  .....
Al
.....
Al
(Click
anywhere on the diagram to enlarge it on a new web page) 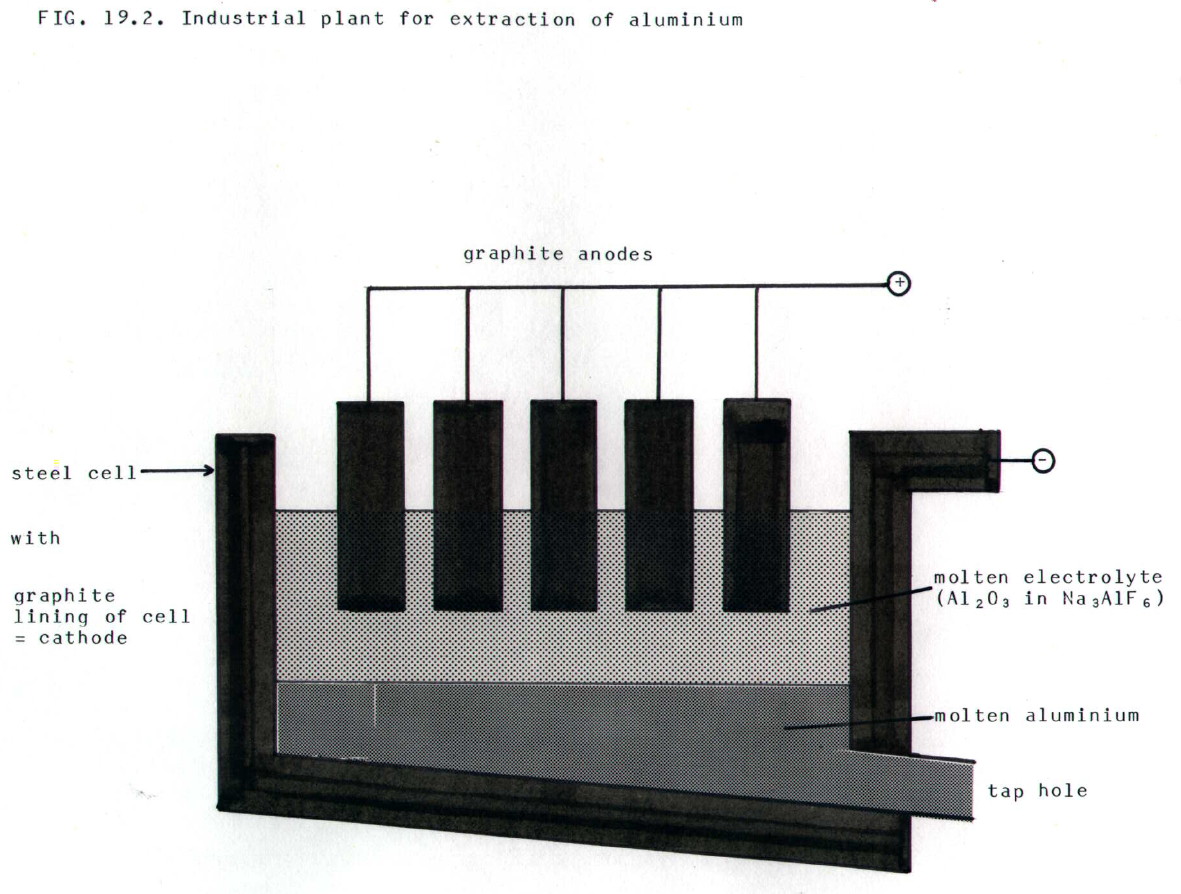
........................ viii) However, aluminium is also the most cost-effective metal to recycle. Recycling 1kg of aluminium saves up to 6kg of bauxite, 4kg of chemical products and 14 kWh of electricity. In fact, according to the Oxford Brookes University Environmental Information Exchange , aluminium is the only packaging material whose value exceeds the costs of collection and processing at recycling centres.
Recycling aluminium
requires only 5% of the energy and produces only 5% of the CO2
emissions as compared with primary extraction from Bauxite. According
to Waste
Watch, the recycling of one aluminium can saves enough energy
to run a television for 3 hours. Sounds like a good idea to me.
19.1.5. Zinc to tin:
External chemical reducing agent: The most commonly used
reducing agent is carbon (e.g. coke) because this is cheap. However,
others are used, as summarised in table 19.1.
| TABLE 19.1. | |||
| Metal | Compound reduced | Reducing agent | Process |
| Zinc | ZnO | coke | blast furnace |
| Iron | Fe2O3, Fe3O4 | coke and CO from coke | blast furnace |
| Nickel | NiO | H2 | |
| Tin | SnO2 | coal | furnace |
| Manganese | Mn3O4 | aluminium | thermite process |
| Chromium | Cr2O3 | aluminium | thermite process |
| Titanium | TiCl4 | sodium | |
| Zinc is sometimes extracted by electrolysis. | |||
19.1.6. Iron: The main ores are Fe2O3
(haematitite), Fe3O4(magnetitite),
and Fe2O3.H2O
(limonite). FeS2 (iron pyrite) is also a common
ore, but seldom used because sulphur impurites in iron make it very
brittle and are difficult to remove. Iron is extracted from the ore in
a blast furnace:
.................. i) The blast furnace (FIG. 19.3.) is charged with (impure) iron ore, coke, and limestone, CaCO3.
................. ii) Hot, dry air is blasted into the bottom of the furnace via tubes (called "tuyères").
................ iii) Carbon monoxide is formed near their inlet point (hottest part of furnace) from coke plus oxygen. Carbon dioxide is also formed both from the coke plus oxygen, and from decomposition of the limestone. However, carbon dioxide is reduced by coke to carbon monoxide in a cooler part of the furnace.
............... iv) When carbon monoxide reaches the top half of the furnace (cooler), it reduces the iron ore to iron. It is the main reducing agent in the furnace.
............... v) As the charge descends in the furnace, remaining ore is reduced by carbon in the lower (hotter) regions.
.............. vi) The iron melts in the lower regions of the furnace, especially because its melting point (normally 1539 °C) is lowered by absorption of carbon. The impure pig iron collects at the bottom of the furnace and is run off.
............. vii) The role of the limestone is to combine with earthy impurities in the ore. It does so near the top of the furnace. This gives a fusible slag of calcium silicate and calcium aluminate which collects on top of the molten iron and which is run off separately. The furnace would soon clog up if limestone were not introduced with the charge. Continuous functioning is an essential to the efficient running of blast furnaces which are very difficult and expensive to re-start.
........... viii) Effluent
gases are used to preheat the air blast, not only because they are hot,
but also because they still contain combustible CO.
(Click
anywhere on the diagram to enlarge it on a new web page) 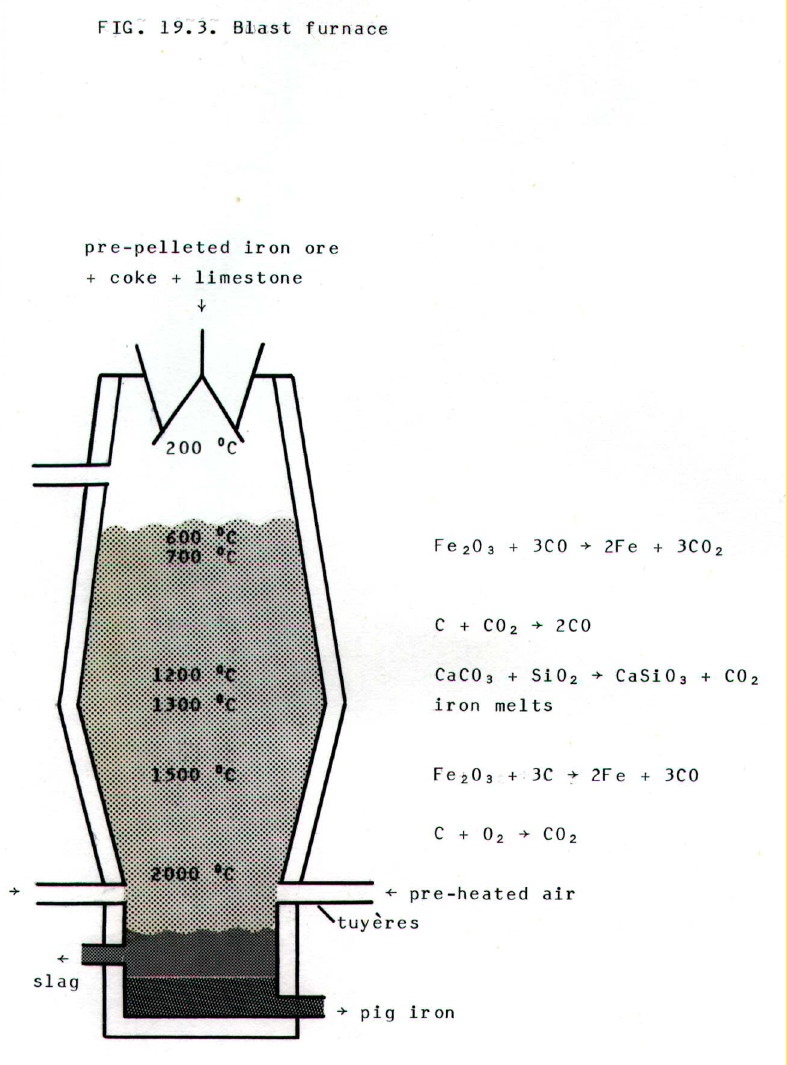
Iron ore and the other raw
materials are relatively cheap, though transportation costs must also
be considered, as well as the cost of further pre-heating of the air
for the furnace, if needed. The ore does not need to be purified and
purification of the metal is relatively easy and cheap.
The efficiency of the process is a balance of:
............. i) the %age extraction of iron from the ore,
............ ii) the consumption of coke and air,
........... iii) the rate at which iron is extracted,
........... iv) the consumption of heat.
The balance is achieved by controlling:
............. i) The percentage of coke in the charge. Too much coke is inefficient in terms of its consumption. Too little coke is inefficient because it will not encourage forward reactions. A slight excess is used.
............ ii) The rate of the air flow. If the rate of flow is too high, excess oxygen will favour carbon dioxide formation and also raise the temperature. High temperatures will discourage the main reactions which are exothermic in the forward direction (sections 8.6. and 10.3.). If the rate of flow is too low, the supply of CO for the main reduction reaction will be reduced, and the temperature may become too low to collect the iron in its molten state.
This is in addition to the effects of temperature on rate of reaction (section 9.7.).
A compromise flow rate
is selected, and the temperature problem is overcome to some extent by
achieving a temperature gradient in the furnace. The reactions
occurring at various temperatures in the furnace can be predicted using
data on the variation of free energy with temperature. This is
summarised in, so called, Ellingham diagrams.
(Click
anywhere on the diagram to enlarge it on a new web page) 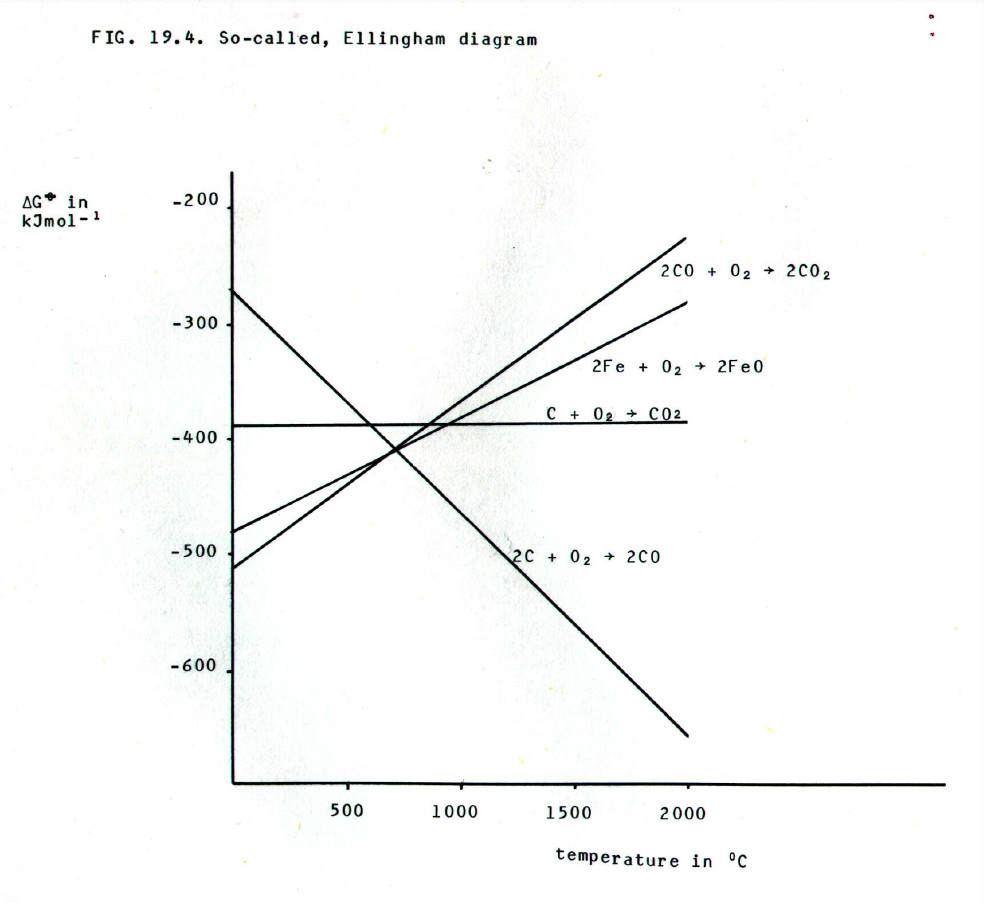
The more negative a  G value for a reaction, the more
likely it is to occur (section 12.5.1. and 12.5.3.). From FIG. 19.4. it
can be seen that below 650°C, the
G value for a reaction, the more
likely it is to occur (section 12.5.1. and 12.5.3.). From FIG. 19.4. it
can be seen that below 650°C, the  G
value for oxidation of CO
G
value for oxidation of CO  CO2 is more negative than that for the oxidation
of Fe
CO2 is more negative than that for the oxidation
of Fe  FeO, but
less negative above 650°C. Thus CO is able to reduce FeO to the metal
below 650°C, but not above it.
FeO, but
less negative above 650°C. Thus CO is able to reduce FeO to the metal
below 650°C, but not above it.
However, similar argument shows that carbon is unable to reduce FeO below 770°C but can reduce it to the metal above 770 °C. Thus, this reaction takes place lower in the furnace.
The reactions occuring in the furnace are complex, and they probably take place in steps, with reduction of FeO being the final step.
19.1.7. Steel: The iron obtained from the blast furnace is hard and brittle due to the impurities it contains (section 18.7.). It is not useful for structural purposes but can be used for heavy castings like drain covers, drain pipes etc.
Iron is converted to steel by burning off carbon impurities in the L.D. process. A water-cooled lance is used to blast oxygen through molten iron in a converter. The carbon is oxidised to CO which is burned off at the mouth of the converter. The purification is extended by two possible modifications to the process:
i) Basic process: When the iron from the blast furnace contains phosphorus, the converter is lined with CaO/MgO (calcined dolomite) and CaCO3 (limestone) is added to the charge.
Calcium oxide from dissociation of the limestone reacts with oxides of silicon and phosphorus to give a fusible slag. There is also some reaction with the lining.
ii) Acidic process: When the pig iron does not contain phosphorus, the converter is lined with silicon dioxide (silica).
Finally, an Fe/Mn/C alloy is added. The manganese reacts with any iron oxides produced during the process, reducing them back to iron. The Manganese oxide passes into the slag. Carbon from the alloy gives a controlled percentage of carbon in the steel (½% mild steel, 1.5% hard steel). In addition, various transition metals may be alloyed with the steel to give it special properties (section 18.7.).
iii) Recycling:
Note also, that every tonne of steel packaging recycled makes the
following environmental savings:
l1.5 tonnes of iron ore.
l 0.5
tonnes of coal.
l 40%
of the water required in production.
l 75%
of the energy needed to make steel from
virgin material.
l 1.28
tonnes of solid waste.
l Reduction
of air emission by 86%.
l Reduction
of CO2
emissions by 80%.
l Reduction
of water pollution by 76%.
To put this into perspective:
Each household uses
approximately 600 steel cans per year
l There
are over 300million cans used per week over the xmas period
l The
value of used steel cans in the waste stream is £28 milllion
l The
recycling rate of all steel packaging is 46%; aluminum has a 23.4%
packaging recycle rate
l There
are over 2.5 billion cans recycled in the UK each year - a saving of
125,000 tonnes of solid waste every year
l All
steel cans contain up to 25% recycled steel
l Two-thirds
of all cans on supermarkets shelves are made from steel
l Recycling
seven steel cans saves enough energy to power a 60-watt light bulb for
26 hours
For more information
visit the Wates Watch online service, waste
online or the Corus Steel
Can Recycling Information Bureau.
19.1.9. Lead and copper, reduction without an external reducing agent: A main copper ore is copper pyrites (CuFeS2) and lead occurs principally as the sulphide.
CuFeS2 is first converted to the sulphide by roasting with air to remove the iron, ultimately as a silicate slag (see equations below). In each case, the sulphide is partially converted to the oxide by roasting in air. The air supply is then cut off, and the temperature is raised, resulting in auto-reduction. The reactions involved are:
2PbS..... +.....
3O2..... .....
2PbO.....
+..... 2SO2
.....
2PbO.....
+..... 2SO2
(PbS..... +..... 2O2.....  .....
PbSO4)
.....
PbSO4)
then strong heat, absence of air:
PbS..... +..... 2PbO.....  .....
3Pb.....
+..... SO2
.....
3Pb.....
+..... SO2
(PbS..... +..... PbSO4.....  .....
2Pb.....
+..... 2SO2)
.....
2Pb.....
+..... 2SO2)
2CuFeS2..... +..... 4O2.....  .....
2FeO.....
+..... 3SO2..... +..... Cu2S
.....
2FeO.....
+..... 3SO2..... +..... Cu2S
stronger heat:
FeO..... +..... SiO2(earthy
material in ore).....  .....
FeSiO3(molten slag floats)
.....
FeSiO3(molten slag floats)
2Cu2S..... +..... 3O2.....  .....
2Cu2O.....
+.....
2SO2
.....
2Cu2O.....
+.....
2SO2
then strong heat, absence of air:
Cu2S..... +..... 2Cu2O.....  .....
6Cu.....
+..... SO2
.....
6Cu.....
+..... SO2
In both cases the metals are purified by using the impure metal as the anode and pure metal as the cathode in electrolysis. Further pure metal is depositied on the cathode. In the purification of lead the electrolyte is lead(II) hexafluorosilicate dissolved in hexafluorosilicic acid, in the case of copper, it is aqueous copper(II) sulphate.
19.1.10. The least reactive elements, such as silver and gold, may occur native. If they do have to be reduced from ores, the reduction is easy. For example, mercury is obtained by roasting the sulphide ore in air or by displacement with iron (filings), and silver is produced by displacement with zinc:
HgS..... +..... O2.....  .....
Hg.....
+..... SO2
.....
Hg.....
+..... SO2
HgS..... +..... Fe.....  .....
Hg + FeS
.....
Hg + FeS
2[Ag(CN)2]-..... +..... Zn.....  .....
[Zn(CN)4]2-..... +..... 2Ag
.....
[Zn(CN)4]2-..... +..... 2Ag
The dicyanosilver(I) ions are the product of a process used to extract silver ions from a low grade ore.
19.2. NON-METALS
19.2.1. Introduction: The methods used for extraction of non-metals also depend partly on their electronegativities, and can be predicted from their positions in the periodic table. The least electronegative non-metals which occur in combination with more electronegative elements, must be extracted by reduction techniques (e.g. hydrogen and phosphorus). The most electronegative usually occur as negative ions which must be oxidised (e.g. the halogens), and inbetween, several non-metals occur in the native state (e.g. sulphur, oxygen, and nitrogen), and these must be separated by physical means.
19.2.2. Reduction techniques: Hydrogen is obtained from natural gas:

Hydrogen is also obtained as a by-product in the manufacture of sodium hydroxide (section 19.3.1.) and as one of the products in the cracking of petroleum (section 26.2.3.).
Phosphorus is obtained from phosphate rock (CaF2.3Ca3(PO4)2). The key reaction occurs in a furnace at 1500 °C with sand and coke:
2Ca(PO4)2.. +..
6SiO2...+..
10C..  ..
6CaSiO3.. +..
P4..
+..
10CO
..
6CaSiO3.. +..
P4..
+..
10CO
19.2.3. Native
non-metals: Diamond and graphite are separated mechanically
from surrounding impurities, nitrogen is separated from air by
fractional distillation of liquid air, and sulphur is obtained from
naturally occurrring deposits under quicksand by pumping superheated
water (170 - 180 °C) down a borehole into the sulphur deposit. The
water is pumped down the outer of three concentric tubes. Compressed
air is pumped down the central tube and this forces water together with
sulphur (which has been melted by the water) up the remaining tube. The
sulphur plus water is run into wooden vats where the sulphur solidifies
as 99.8% pure solid.
(Click
anywhere on the diagram to enlarge it on a new web page) 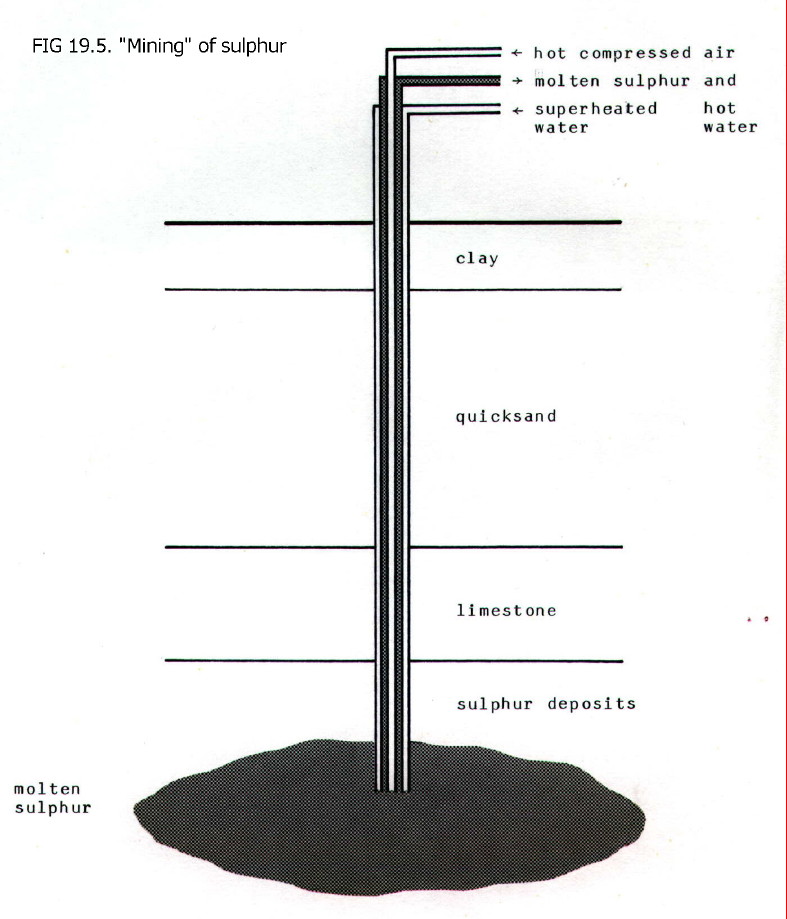
19.2.4. Halogens:
Fluorine is extracted from fluorspar (CaF2) by a
complex procedure ending with electrolysis of potassium fluoride
dissolved in hydrogen fluoride. Chlorine is extracted mainly from
naturally occurring rock salt (NaCl) by electrolysis in the same
processes which are used to extract sodium and other electropositive
metals, and to manufacture sodium hydroxide (sections 19.3.1. and
19.1.3.).
Bromine is extracted from sea water by displacement with chlorine in acid conditions, and iodine is extracted from Chilean salt deposits by reduction of IO3- ions by sulphur dioxide to iodide, then a redox reaction between the iodide and more iodate(V) ions produces the iodine (question 19.3.).
19.3. COMPOUNDS
19.3.1. Sodium hydroxide, like sodium metal (section 19.1.3.) is produced by electrolysis of naturally occuring sodium chloride. However, in this case, aqueous rather than fused sodium hydroxide is electrolysed, and the cathode is a flowing mercury cathode. The ions present are Na+, Cl-, H+, and -OH. The electrode reactions are:
Cathode:.... Na+.... +....
e-....  ....
Na
....
Na
This occurs despite the fact that hydrogen is less electropositive than sodium, because it is difficult to discharge hydrogen ions at a mercury surface (section 11.4.2.), and possible to discharge sodium ions, because the sodium dissolves in the mercury.
Anode:.... Cl-.... -....
e-....  ....
.... 
.......................... 2 ....
....  ....
Cl2
....
Cl2
This occurs in
preference to the discharge of hydroxide ions, despite what might be
predicted from their relative positions in the electrochemical series,
because the chloride ions are much more concentrated. Note that sodium
chloride solution must flow through the electrolytic cell at such a
rate that the chloride ion concentration is maintained above a critical
level. Otherwise, hydroxide ions will be discharged, and this will
contaminate the chlorine produced in the process with oxygen.
(Click
anywhere on the diagram to enlarge it on a new web page) 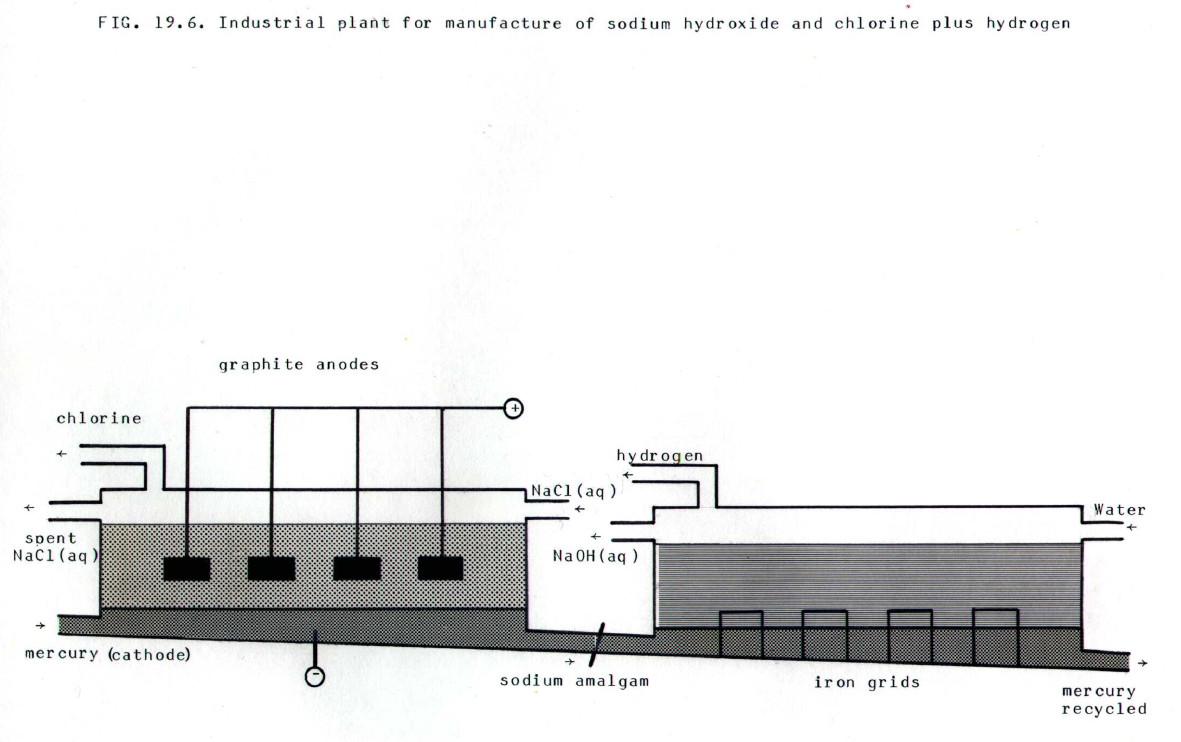
The mercury amalgam is treated
with water in the presence of iron grids which make contact with the
amalgam and the water. This creates an electrochemical cell (section
11.7 to 11.9.):
Na,Na+(aq) / Hg | H+(aq),H2(g) / Fe
in which the cell reaction is:
2Na(amalgam).... +....
2H+(aq)....  ....
2Na+(aq).... +....
H2(g)
....
2Na+(aq).... +....
H2(g)
This leaves sodium and hydroxide ions in solution. The water is evaporated, and sodium hydroxide solidifies on cooling.
19.3.2. The effect of economic and chemical factors is well illustrated by the manufacture of ammonia.
The essential reaction is an exothermic equilibrium reaction:
N2(g).... +....
3H2(g)....  ....
NH3(g)
....
NH3(g)
Applying Le Chatelier's principle (section 8.6.) it can be predicted that formation of ammonia will be favoured by:
................... i) low temperatures
.................. ii) high presures
However, low temperatures are also undesirable because they will give a low rate of production, and high pressures are undesirable because the equipment is expensive.
A compromise temperature of around 550°C is selected and an iron catalyst containing iron(III) oxide and aluminium(III) oxide promoters is used to counteract the effect on rate. The choice of catalyst is influenced by economic factors, since more expensive transition metals like platinum are actually more effective.
A compromise pressure of around 200 atmospheres is selected, though in some cases pressures of up to 1,000 atmospheres are used. An approximately 10% yield is obtained but the gases are re-cycled after removal of ammonia by cooling.
19.3.3. Nitric(V) and sulphuric(VI) acids: The processes for manufacture of these acids are summarised in the equations below:
i) Nitric(V) acid:
....................
900°C/atmos. press./Pt,Rh gauze
4NH3(g).. +...5O2(g)....  ....
4NO(g)..
+.. 6H2O(g).... Yield = 95%.
....
4NO(g)..
+.. 6H2O(g).... Yield = 95%.
The heat produced mantains the temperature of the gauze if the flow of gases is correctly controlled. The mixture is cooled, then:
2NO(g).... +....
O2(g)....  ....
N2O4(g)
....
N2O4(g)
The nitrogen(IV) oxide is bubbled with air into water:
2N2O4(g).... +....
2H2O(l).... +....
O2(g)....  ....
4HNO3(aq)
....
4HNO3(aq)
ii) Sulphuric(VI) acid:
S(s).... +....
O2(g)....  ....
SO2(g)
....
SO2(g)
or:
4FeS2(s).. +..
11O2(g)...  ...
2Fe2O3(s).. + ..
8SO2(g)
etc.
...
2Fe2O3(s).. + ..
8SO2(g)
etc.
N.B. SO2 prepared from pyrites contains arsenic(III) oxide which must be removed to avoid poisoning the catalyst in the next stage:
.................................
450°C/V2O5
2SO2(g).... +....
O2(g)........  .......
2SO3(g)....
Yield = 96%.
.......
2SO3(g)....
Yield = 96%.
The sulphur(VI) oxide is dissolved in continuously diluted 98% sulphuric acid:
SO3(g).... +....
H2O(l)....  ....
H2SO4(aq)
....
H2SO4(aq)
If the gas were dissolved in water it would be difficult to control the highly exothermic reaction.
If the sulphuric acid is not continuously diluted and removed, a solution of sulphur(VI) oxide in anhydrous sulphuric acid is obtained. This is known as fuming sulphuric acid, or oleum, and it is used in some manufacturing processes such as the production of detergents (sections 22.11. and 26.4.2.).
19.4. FINAL NOTE ON ECONOMIC FACTORS
We have already mentioned abundance, accessibility and cost of raw materials as controlling factors in the economics of industrial processes. Transportation costs and cost of power, heating, and equipment have also been considered.
Other factors which have an important effect on the economics and location of industrial plants include:
......................i) Percentage yield: This is the actual yield of desired product expressed as a percentage of the maximum yield possible. The maximum yield is calculated using the stoichiometric equation(s) for the reaction(s) that produce the product.
Percentage
yield =
actual yield
x 100
............................theoretical
yield
Losses can result from incomplete conversion, losses when the desired product is collected and/or purified and so on.
.....................ii) Atom economy is a measure of percentage of the atoms in the raw materials (reactants) which actually end up in the desired product as opposed to by product(s). For example, even if the yield when
Cu2S..... +..... 2Cu2O.....  .....
6Cu.....
+..... SO2
.....
6Cu.....
+..... SO2
were 100%, which it is not, not all the raw materials would be converted into the desired product, copper, because there is a by product, SO2.
% Atom economy =
molar mass of desired product
x 100
...............................sum
of molar massess of all *products
*or reactants, given the sum of the molar masses is the same for both reactants and products and given that the importance of atom economy is as a measure of the proportion of reactant atoms which end up in the desired product.
Both yield and atom economy are measures of waste (or potential waste - see iii below). From a sustainability point of view, both a high percentage yield and a high atom economy are desirable (again subject to iii below).
................... iii) The value of by-products. This may be advantageous when more than one product has commercial value (e.g. chlorine/sodium hydroxide/hydrogen production), or disadvantageous when disposal of by-products without damage to the environment adds to the cost (chapter 28).
.................. iv) Cost of maintaining the plant.
................. v) The cost of personnel from unskilled to managerial. This varies widely with geographical location, even within the UK, let alone worldwide.
................ vi) Cost of land for building the plant, as well as closeness to transport, populated areas etc.
................ vii) Demand for the product (i.e. the dreaded market forces) which amongst other things will determine the level of production.
19.5. QUESTIONS
1) Discuss the economics of aluminium extraction. Why is electrolysis used rather than chemical reduction of the ore by an electropositive metal?
2) Judging from the reducing agents used to extract the elements in TABLE 19.1 from their ores, speculate about the relative costs of the metals listed. Explain your reasoning, bearing in mind the other factors that will influence the final cost of the metal, and give a final order.
3) Write balanced equations for the processes described for the extraction of bromine and iodine.
4) Speculate about the logic of an industry which does not generally use sodium to extract other electropositive metals from their ores, but which does use chlorine to extract bromine from seawater, given that sodium and chlorine are both obtained from the same source.
5) Speculate about the economic and chemical factors which determine the conditions chosen for the manufacture of nitric(V) and sulphuric(VI) acids.
Unless otherwise stated, all materials in this web version of chapter 19 are © 2007 Adrian Faiers MA (Oxon) MCIPR

What 's the connection between a dozen eggs and
a garden mole?

Answer: Not a lot, really, but see Chapter 1