Recommended by:

Top 20 UK science resources,
The Tutor Website
Recommended by:

Rated:

2010
Listed on the science, engineering and technology section of

'providing you with access to the very best Web resources for education and research, evaluated and selected by a network of subject specialists.'
(Please note that intute closed in July 2011)
Section 3: Organic
CHAPTER 20: TWELVE
ESSENTIAL REACTION MECHANISMS
NB This chapter has now been
updated to improve browser compatibility.
Please
use the 'send email' link at the top right hand corner of this page to
report any problems.
20.1
INTRODUCTION
Traditionally, organic chemistry comprised yet another tedious list of reactions to be learned. Mechanisms have changed all that.
An understanding of twelve basic reaction types makes A-level organic chemistry one of the easiest and most satisfying parts of the syllabus. The reaction types are the door to prediction (c.f. section 13.1.). Even so, there are still people who prefer to learn the tedious list of reactions and then learn their mechanisms as some kind of masochistic extra.
The reason is simple. Learning a reaction parrot-fashion requires little mental work and brings instant reward. Understanding mechanisms takes a lot of mental work and initially there is little impact on the huge list of organic reactions which is, admittedly, included in the syllabus.
However, when the parrot is burning the midnight oil, learning its two hundredth reaction, the thinking student is out having a good time, confident that he or she can predict what would happen in "reaction 200". Laziness can make you very busy.
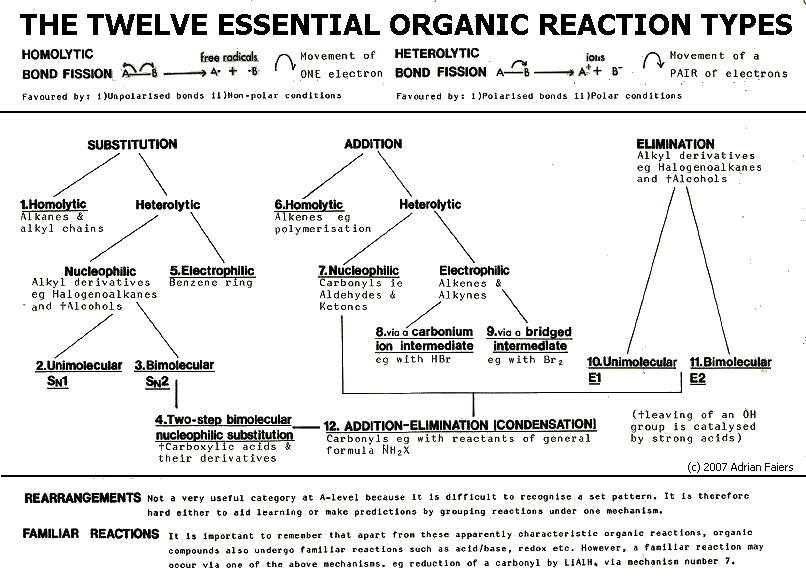
So what are these
twelve reaction types? They are summarised in FIG 20.1., and described
in sections 20.3. onwards. From the descriptions, it will become clear
that the key to understanding organic mechanisms is electrostatic
attraction and repulsion (c.f. section 13.1.).
FIG
20.1 (Click on diagram to enlarge on a new web page)
20.2 BOND FISSION
A bond involves two bonding electrons, and when the bond breaks, there are two possible fates for the pair of electrons. In other words there are two main ways in which bonds can break.
20.2.1. In homolytic bond fission each of the two bonded atoms or groups holds on to one of the electrons. This produces, so-called, free radicals. When a bond between two atoms breaks homolytically, the free radicals are nothing more, and nothing less, than free atoms. Not surprisingly, free radicals are very reactive and therefore tend to be short-lived.
In Homolytic reactions, electrons move in "ones". For example, the two electrons which make up a bond that is breaking homolytically, move independently of each other.
In diagrams of reaction mechanisms, movement of one electron is shown by a half-headed curly arrow. (FIG 20.1.)
Note also that the
"dot" on a symbol for a free radical represents an unpaired electron. (A and
and  B in FIG
20.1.)
B in FIG
20.1.)
20.2.2. In heterolytic bond fission one of two the bonded atoms or groups holds on to both the bonding electrons, thus becoming a negative ion. The other atom or group becomes a positive ion.
In heterolytic
reactions, electrons move in pairs. For example, the two electrons
which make up a bond that is breaking heterolytically, move together.
In diagrams of reaction mechanisms, movement of a pair of electrons is
shown by full-headed curly arrow. (FIG 20.1.)
20.2.3. Charge
often gives rise to confusion in heterolytic reaction mechanisms.
When a species has a single negative charge, that means it has one
extra electron, i.e. there is one more electron in the ion than there
are protons in the nucleus (or nuclei in a more complex ion such as ClO4-)
However, heterolytic mechanisms often show a curly arrow (movement of a pair of electrons) coming from a single negative charge. Moreover, despite the apparent loss of two electrons, the resultant species does not acquire a positive charge. There are two things to say.
First, the extra electron which gives an ion its negative charge is always present as one of a pair of electrons in an atomic orbital. It is the pair which moves.
Second, the curly arrow rarely represents total loss of a pair of electrons. Usually, the electron pair forms a covalent bond. In other words, the species usually only loses control of a pair of electrons.
The final overall charge may be zero, because the bond is formed either with a positive ion, or with a species which simultaneously loses, or loses control of other electrons. (E.g. mechanisms 2 and 3 below.)
Thus it is the relative numbers of electrons and protons in a system which determine its charge.
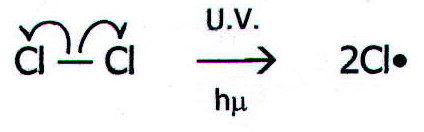
20.3. MECHANISM 1: HOMOLYTIC SUBSTITUTION
e.g. Chlorination of methane in UV light
20.3.1. i)
Initiation: The absorption of ultra-violet light causes the
chlorine to split into free radicals:
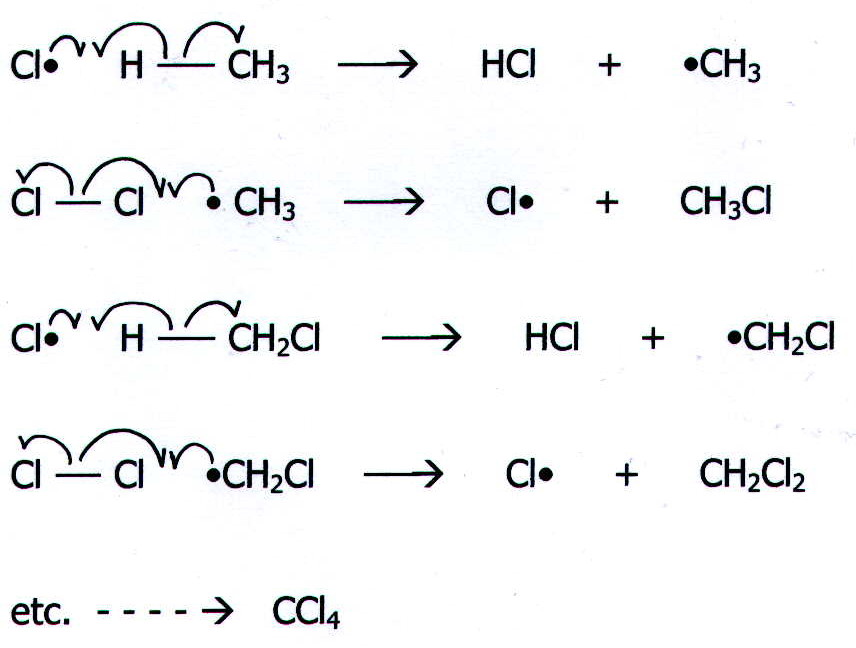
ii) Propagation Each of these steps produces a
reactive free radical and thus reaction continues.
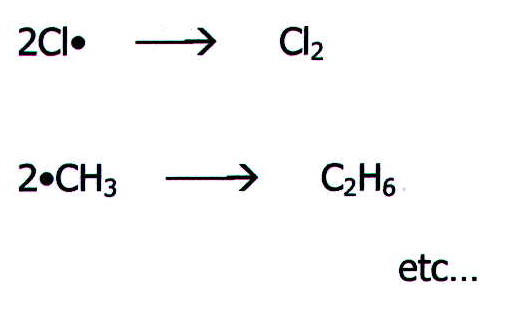
iii) Termination Whenever two free radicals
collide and react, they terminate reaction chains. As reactants are
depleted, so termination reactions bring an end to overall reaction.
20.3.2. Chain reactions. Not all homolytic
reactions are chain reactions. For example, the catalytic hydrogenation
of ethene is homolytic but not a chain reaction.
Moreover, in chain
reactions each of the propagation steps is exothermic or close to
exothermic. The following reaction is endothermic:
![]()
and methane does not react with bromine under the same conditions that
it reacts with chlorine (section 9.11.1.).
20.4. MECHANISM 2: UNIMOLECULAR, NUCLEOPHILIC SUBSTITUTION. (SN1)
Example: The
reaction of a tertiary haloalkane with aqueous potassium hydroxide.
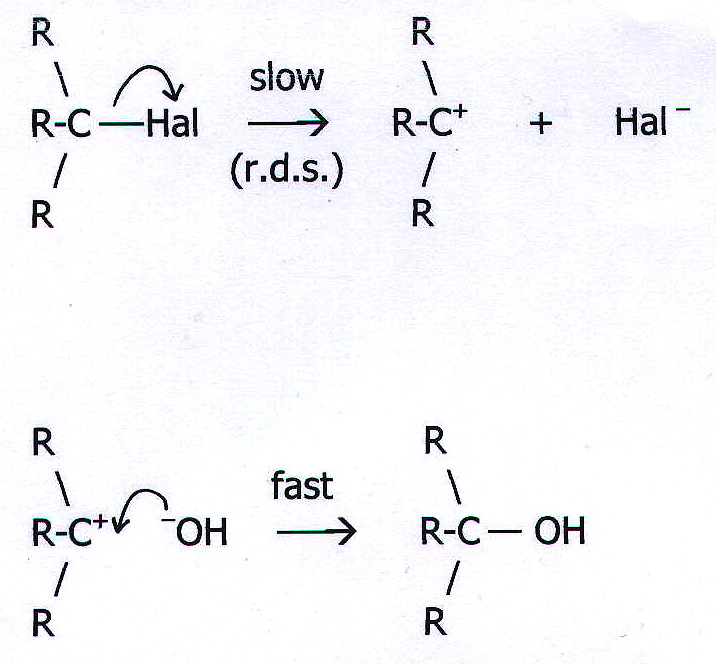
Note that the head of the curly arrow in the first equation points at
the halogen atom. This represents a transfer of electrons from the
C-Hal bond to the halogen.
However, in the second equation the head of the curly arrow does not point at the positive carbon atom. This is because the electrons are not transferred to the carbon atom. The direction of the arrow head represents the transfer of the -OH electrons into a bond between the carbon and the oxygen.
20.5. MECHANISM 3: BIMOLECULAR NUCLEOPHILIC SUBSTITUTION. (SN2)
Example: The
reaction of a primary haloalkane with potassium hydroxide solution.
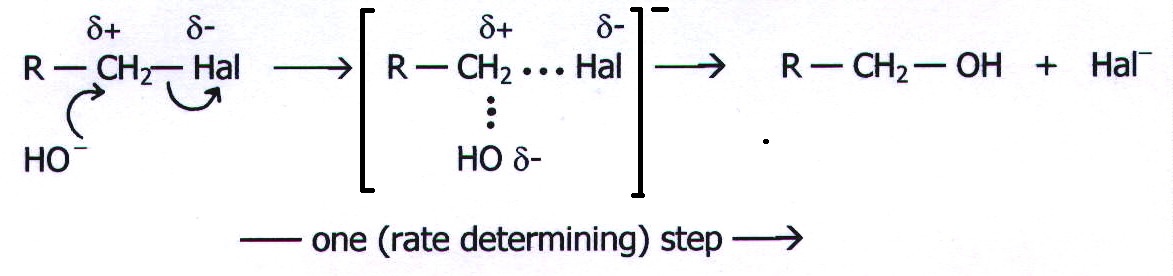
Note that the unimolecular
reaction occurs in two steps, whereas the bimolecular reaction occurs
in one step.
20.6. NUCLEOPHILES
From the above reaction mechanisms, it can be seen that nucleophiles are reactants which attack regions of exposed nuclear charge (hence the name: they are nucleus lovers) and provide at least one pair of electrons for bond formation with the attacked species.
They can be: negative
ions such as halide ions, hydroxide ions, nitrile ions, etc.
or neutral molecules with one or more available lone pairs such as

20.7. MECHANISM 4:
TWO-STEP BIMOLECULAR NUCLEOPHILIC SUBSTITUTION.
Example: The
reactions of carboxylic acids and their derivatives with nucleophiles
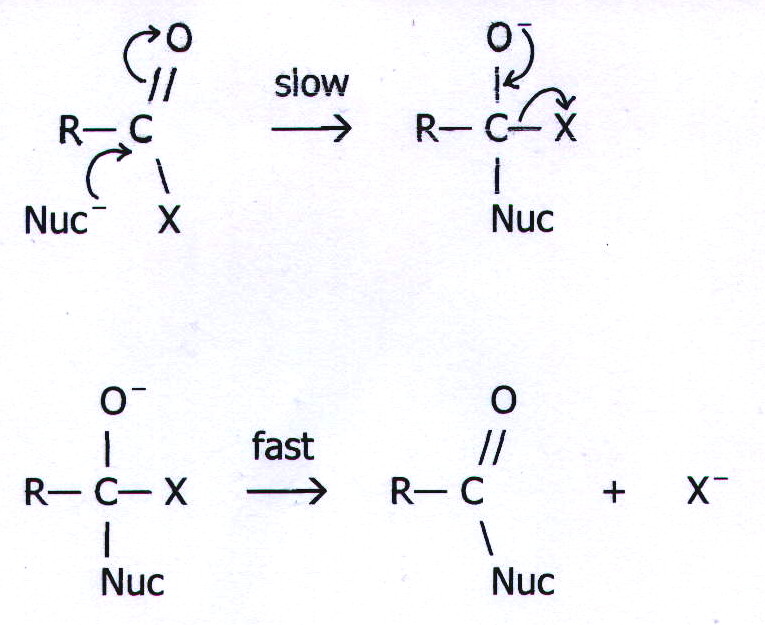
20.8.
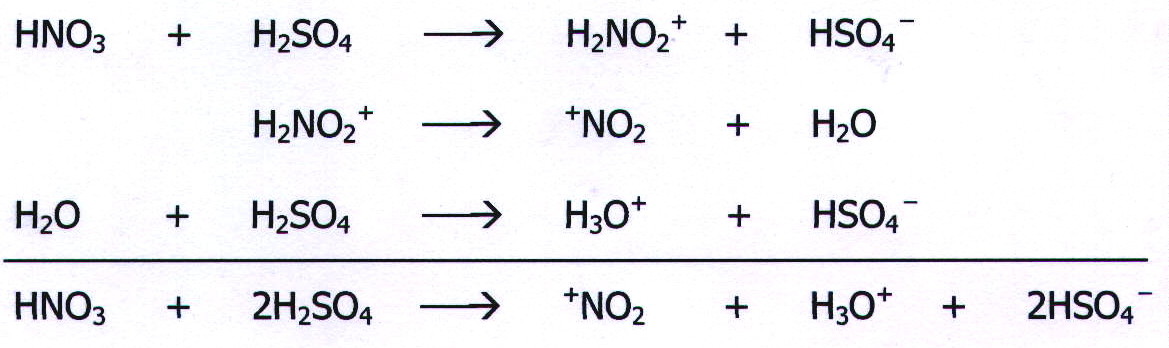
MECHANISM 5: ELECTROPHILIC SUBSTITUTION
Example: The
nitration of benzene by concentrated nitric acid dissolved in
concentrated sulphuric acid.
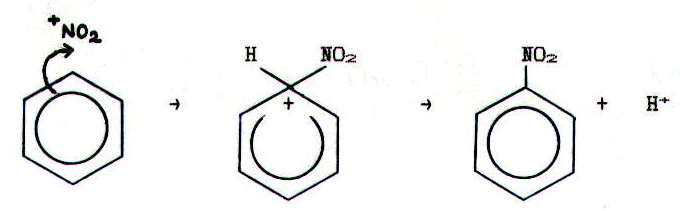
The electrophile in this case (+NO2) is produced by reaction between nitric acid and sulphuric acid:
20.9. ELECTROPHILES
The name electrophile means electron lover. Electrophiles are reactants which attack regions of high electron density (such as multiple bonds) and accept at least one pair of electrons in bond formation with the attacked species.
They can be: positive
ions such as NO2+
...................polarised
molecules such as H-Cl, H-Br, etc
...................polarisable
molecules such as bromine in the presence of a double bond or halogens
in the presence of a halogen carrier like aluminium(III) chloride.
20.10. NUCLEOPHILIC OR ELECTROPHILIC ATTACK?
20.10.1. The distinction between electrophilic and nucleophilic reactions may seem somewhat arbitrary. When a reactant is attacked by a nucleophile, it is itself behaving as an electrophile. Equally, when a reactant is attacked by an electrophile, it is equally valid to regard the attacked species as a nucleophile attacking the first reactant!
20.10.2. Deciding factors are rather arbitrary: If one reactant is organic and the other is inorganic, it is common to regard the organic reactant as the species undergoing attack. If both are organic, it is often the larger molecule which is regarded as undergoing attack.
Alternatively, it is common to regard the main functional group under consideration in a particular line of study as the group undergoing attack. For example, when studying primary alcohols you may consider the small number of nucleophiles which attack them. When studying acid chlorides you may consider the larger number of nucleophiles which attack them - and find that primary alcohols are included in that number.
20.11. SUBSTITUTION
From the above reactions we are now in a position to describe substitution. In substitution reactions, atoms or groups are added to the reacting species in place of atoms or groups which are lost. This may seem like a clumsy description, but here it is written in this way for direct comparison with later descriptions of addition and elimination.
20.12. MECHANISM 6: HOMOLYTIC ADDITION
20.12.1. Example 1: The reaction of an alkene with HBr under conditions which produce free radicals.
Conditions which produce free radicals include:
1. Ultra-violet light.
2. The presence of peroxides and other chemical initiators.
3. Autoxidation. This is most likely to have occured in old samples of
a reactant.
Like homolytic substitution, this is a free radical chain reaction and
it occurs in three stages.
i) Initiation
![]()
![]()
ii) Propagation
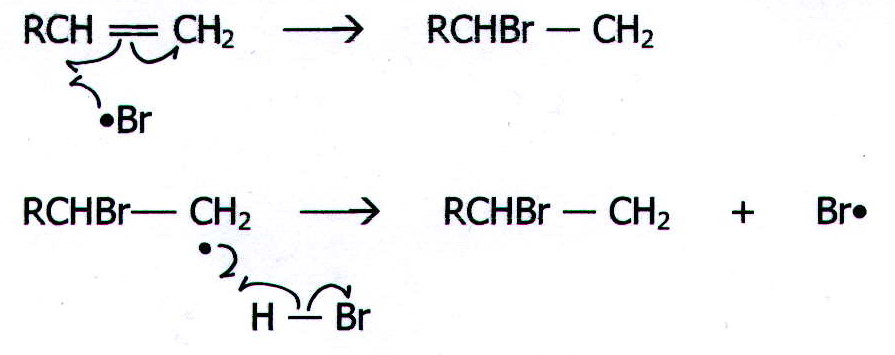
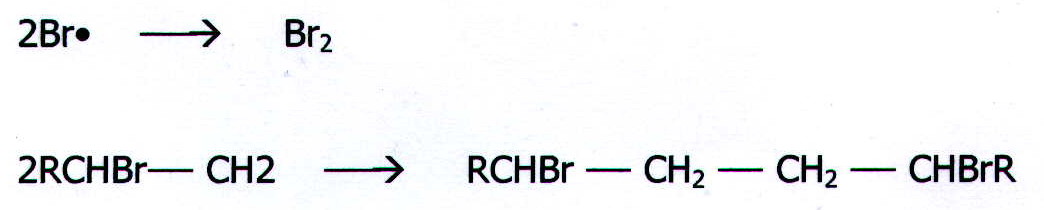
iii) Termination
Free radicals combine
in pairs, e.g.
Note that this mechanism allows HBr to add the other way round from that predicted by Markownikoff's rule. (See mechanism 8.)
20.12.2. Example 2. Polymerisation reactions: the reactions of alkenes in conditions which produce free radicals, but the absence of a second reactant.
i) Initiation
![]()
ii) Propagation
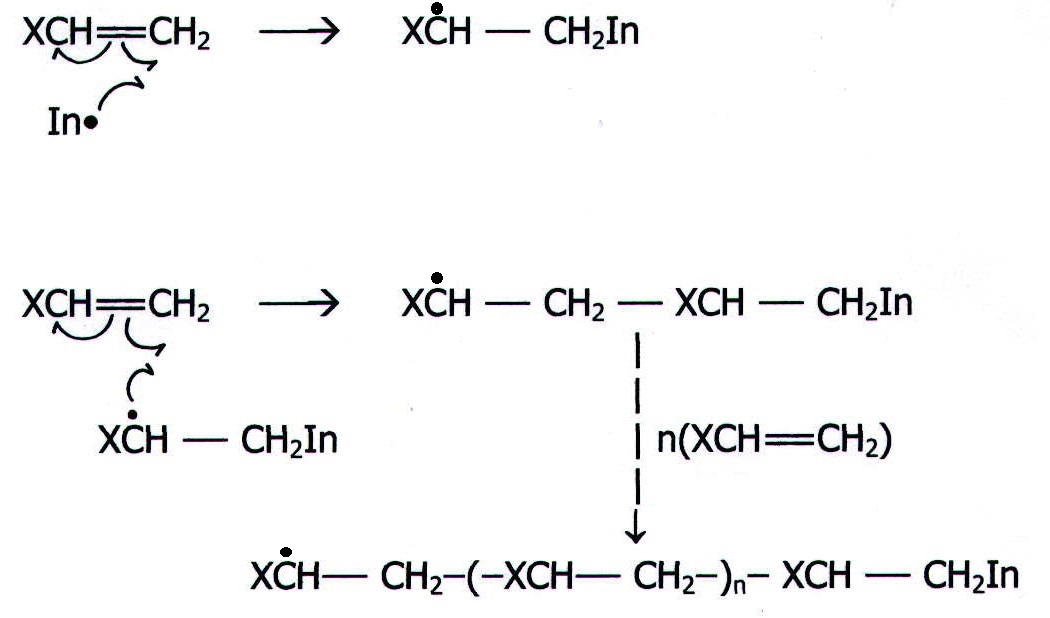
iii) Termination
Ends combine in pairs,
or disproportionation between ends may occur:
![]()
or:
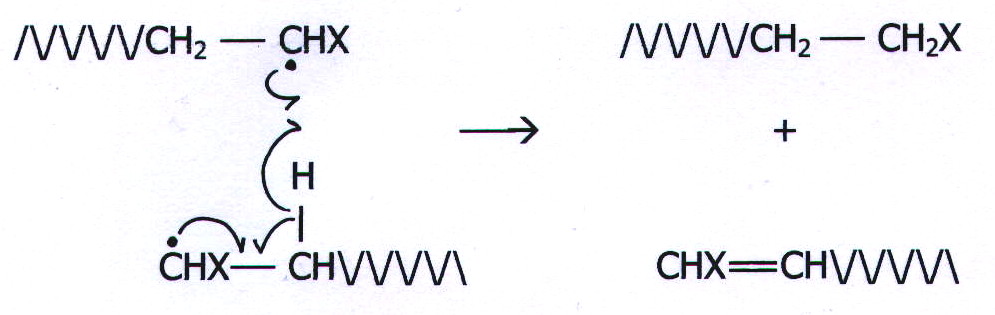
20.12.3. Examples of addition polymers
The above reactions can produce a wide range of well known polymers as summarised in the table below.
|
TABLE 20.1. Polymers produced
by homolytic addition
reactions of alkenes and their derivatives. Trivial names are shown in
brackets. |
||
|
X GROUP |
MONOMER |
POLYMER |
|
- H |
ethene |
polythene |
|
NB The characteristics of
polythene depend very much on the conditions under which the polymerisation is carried out,
temperature, pressure, catalyst etc. |
||
|
-
Cl |
chloroethene |
polychloroethene |
|
- C6H6 |
|
polyphenylethene |
|
- COOH |
|
|
20.13.
MECHANISM 7: NUCLEOPHILIC ADDITION
Example: Addition of nucleophiles to carbonyl compounds
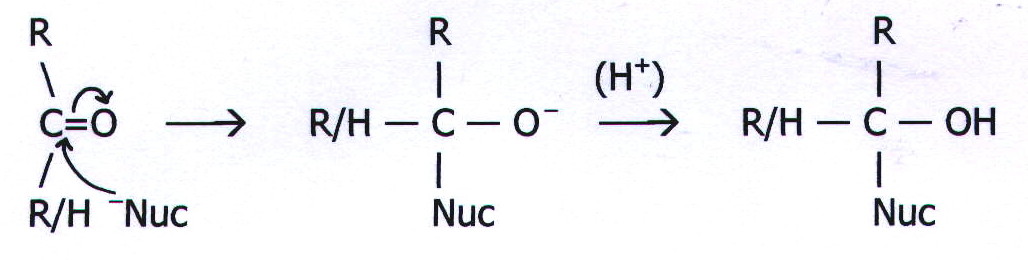
20.14. MECHANISM 8: ELECTROPHILIC ADDITION VIA A CARBONIUM ION INTERMEDIATE.
Example: Reaction of propene with HBr in the absence of free radicals.
20.14.1. Markownikoff's rule. In the absence of free radicals, the main product is predicted by Markownikoff's rule which states that: When an unsymmetrical molecule (such as HBr) adds to an unsymmetrical alkene (such as propene), the more electropositive part of the adding molecule (H in HBr) adds to the least substituted carbon atom (=CH2 in propene) ie, to the carbon atom with most hydrogens already attached to it.
Markownikoff's rule offers no understanding of how this happens. The reaction mechanism does.
20.14.2. The
mechanism.
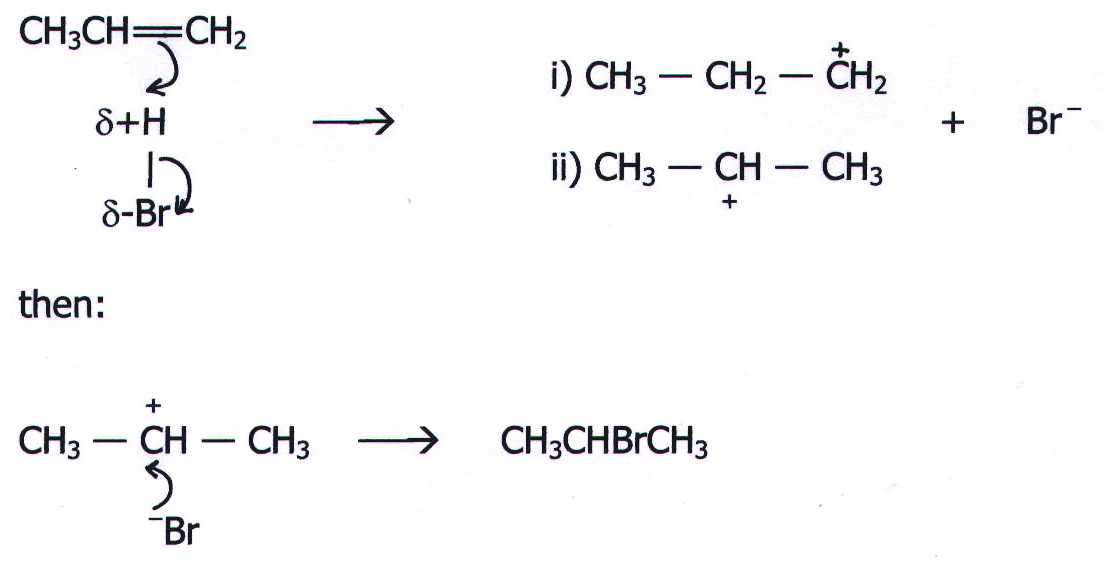
In the first stage of this reaction there are apparently two possible intermediates (i and ii). However, the formation of the secondary carbonium ion (ii) is more likely than the formation of the primary carbonium ion (i) because it involves less pulling away of electrons from the positive centre.
In terms of stability, there are two alkyl groups attached to the positively charged carbon atom in the secondary carbonium ion, as opposed to one in the primary carbonium ion. The alkyl groups exert an electron pushing effect (positive inductive effect) which reduces the concentration of positive charge. The effect is obviously twice as great in the secondary carbonium ion. (See the section on inductive effects in chapter 21.)
20.15. MECHANISM 9: ELECTROPHILIC ADDITION VIA A BRIDGED INTERMEDIATE.
Example:
Reaction of ethene with bromine.
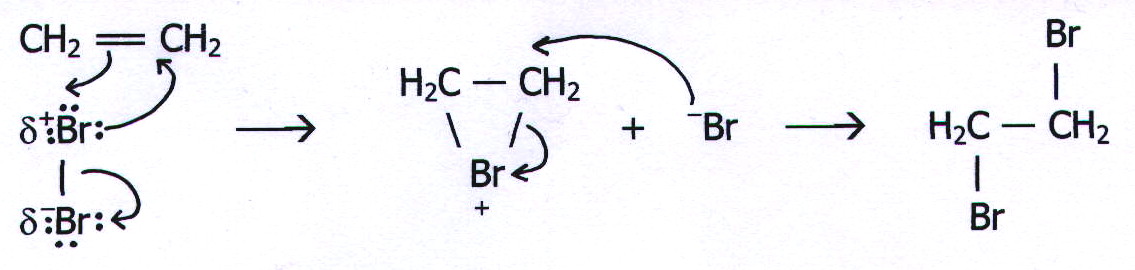
There are several unusual things about this mechanism. The first is the nonsense which is often talked about it.
It is often said that the high concentration of electron density in the alkene double bond polarises the bromine molecule and as a result attracts the positive end. This is ridiculous: repulsion causes attraction! It is more reasonable to say that the high electron density of the double bond attracts the bromine nucleus through the bromine electron cloud, simultaneously polarising the molecule.
The second unusual thing about this mechanism is why it was suggested in the first place. Mechanism 8 seemed perfectly adequate. However, there is some evidence to suggest that, in stage two of the reaction, Br- attacks from the other side of the C-C bond.
Obviously this is impossible to tell in the case of ethene because there is free rotation about the C-C bond in the product.
However:
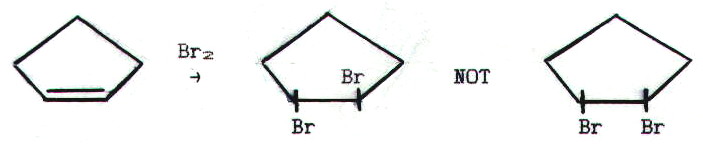
The mechanism also helps us to understand how it is that alkynes are often less reactive than alkenes. With their higher electron density, we might expect alkynes to attract electrophiles more strongly and react with them more quickly. Often they do not.
This can be understood when it is realised that, in the case of addition across a triple bond, the bridged intermediate is more difficult to form: the bromine must fit across a shorter, double bond; also, sp2 bond angles are more distorted. See if you can work out what that second statement means in terms of electron repulsion.

20.16. ADDITION
We are now in a position to describe addition. In addition reactions, atoms or groups are added to the reacting species without any being lost.
20.17. MECHANISM 10: UNIMOLECULAR ELIMINATION (E1).
Example:
Reaction of a tertiary haloalkane with potassium hydroxide in a mixture
of ethanol and water.
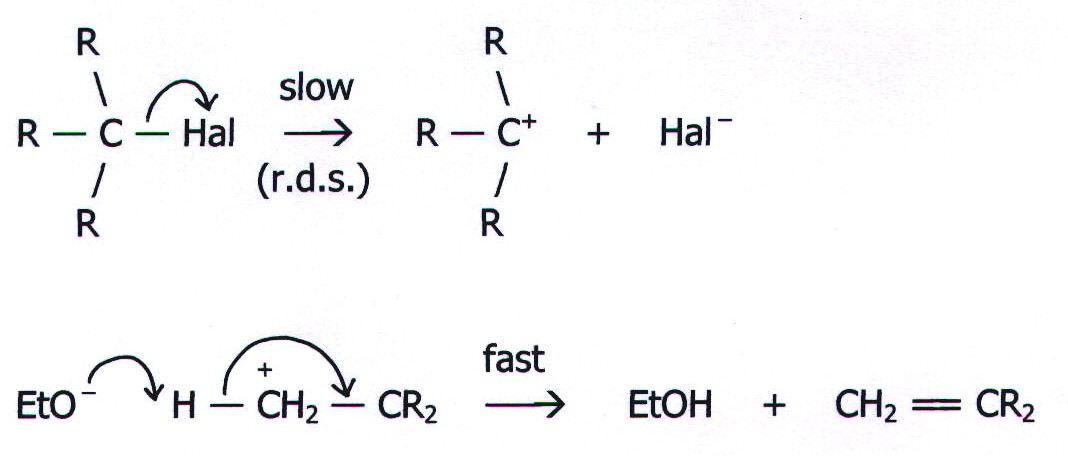
Note that the first stage of this example is identical to the first
stage of the SN1 example reaction. Under many
conditions, substitution and elimination are in competition, and this
will be discussed in the next chapter
20.18. MECHANISM 11: BIMOLECULAR ELIMINATION (E2).
Example:
Reaction of a secondary haloalkane with ethanolic potassium hydroxide.
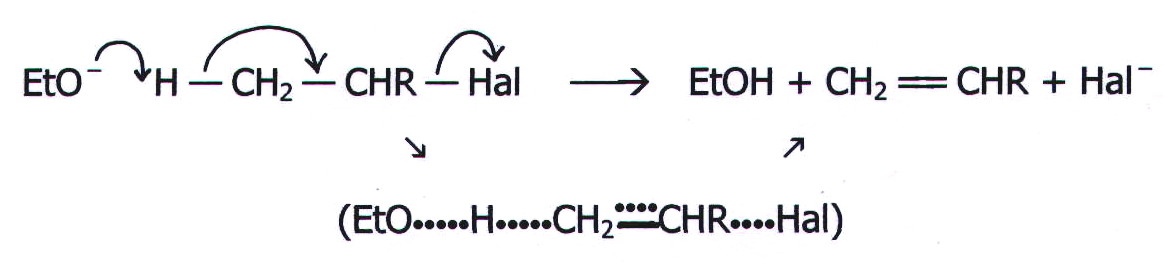
20.19. ELIMINATION
We are now in a position to describe elimination. In elimination reactions, atoms or groups are lost without any being added.
20.20. MECHANISM 12: ADDITION-ELIMINATION (CONDENSATION) REACTIONS.
Example: The reactions of carbonyl compounds with nucleophiles of general formula :NH2X.
There is some similarity between these reactions and two-step bimolecular nucleophilic substitution reactions (mechanism 4), but they should not be confused (as they often are).
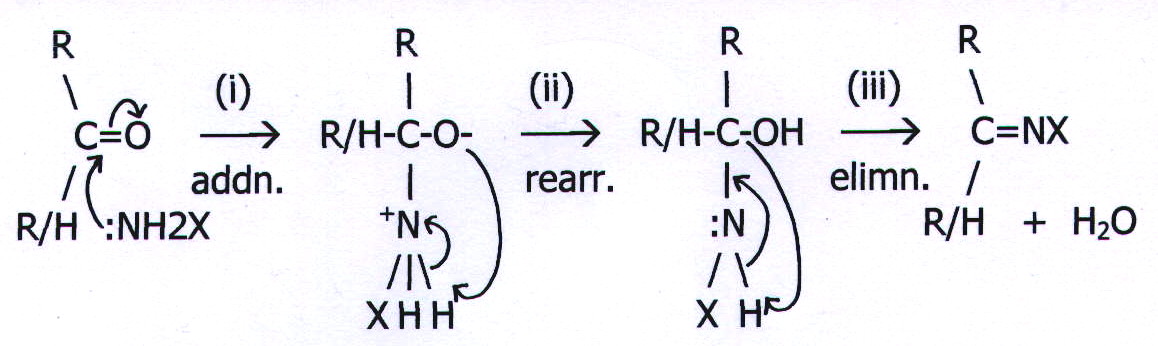
20.21. OTHER TYPES OF REACTION.
20.21.1. REARRANGEMENTS
Stage ii of mechanism 12 is a rearrangement. There are many reactions which can be described in this way, but it is not a very useful category at this level because there is considerable variation between one rearrangement mechanism and another. Without any easily recognised patterns it is difficult to make predictions - one of the main reasons for understanding mechanisms.
20.21.2. FAMILIAR REACTIONS
The above mechanisms have hopefully roused a little enthusiasm. If they have, beware of one danger. These apparently characteristic organic reactions are not the only types of reaction undergone by organic compounds.
Apart from other characteristic organic reactions which are not included in the 12 categories above, there are more familiar reactions undergone by organic compounds such as: acid/base reactions, redox reactions etc.
In your enthusiasm for predicting reactions from the above mechanisms, do not forget the familiar.
However, it is also true that some familiar reactions occur via the above mechanisms. E.g. When carbonyl compounds undergo reduction by LiAlH4 they do so via mechanism 7.
20.22. QUESTIONS
1) Referring only to
the structures given in chapter 27, predict the main types of reaction
undergone by each class of organic compound.
2) Describe in words the movements of electrons which occur in
mechanisms 1 to 4.
3) Given the description of homolytic bond fission in section 20.2.1.,
write a definition of free radical.
4) Explain how a free radical mechanism favours "anti-Markownikoff"
addition.
5) Comment on the following statements:
i) Organic
compounds containing multiple bonds are generally regarded as
nucleophiles.
ii) Propene is susceptible to attack by electrophiles.
iii) Haloalkanes undergo nucleophilic attack and also behave as
electrophiles.
iv) When a nucleophile collides with an acid chloride molecule, it
always collides with the region of exposed nuclear charge, and this
leads to a substitution reaction.
6) What are the key similarities and differences between mechanisms 4
and 12 in this chapter?
Unless otherwise stated, all materials in this web version of chapter
20 are © 2007 Adrian Faiers MA (Oxon) MCIPR

What 's the connection between a dozen eggs and
a garden mole?

Answer: Not a lot, really, but see Chapter 1