Recommended by:

Top 20 UK science resources,
The Tutor Website
Recommended by:

Rated:

2010
Listed on the science, engineering and technology section of

'providing you with access to the very best Web resources for education and research, evaluated and selected by a network of subject specialists.'
(Please note that intute closed in July 2011)
Section 3: Organic
CHAPTER 23: SUMMARY
OF SOME LABORATORY PREPARATIONS
NB This chapter has now been
updated to improve browser compatibility.
Please
use the 'send email' link at the top right hand corner of this page to
report any problems.
23.1. INTRODUCTION
Most of this chapter (section 23.3.) should be unnecessary. By this stage you should be able to predict the best methods for preparing particular organic compounds.
For example, if you are required to prepare an alcohol, you should think like this:
 I need to introduce an -OH group
I need to introduce an -OH group
 -OH
groups are nucleophiles
-OH
groups are nucleophiles
 What groups can easily be
nucleophilically replaced in a carbon chain?
What groups can easily be
nucleophilically replaced in a carbon chain?
 Answer: halogen groups.
Answer: halogen groups.
 It should therefore be possible
to prepare alcohols by reacting haloalkanes
It should therefore be possible
to prepare alcohols by reacting haloalkanes
...with alkali.
 It is.
It is.
Precise methods are determined by:
i) The conditions
required for reaction (for which you should now have a feel).
ii) The final mixture produced by reaction
A few methods of reaction and methods of isolation/purification are discussed below in section 23.2.
23.2. METHODS OF REACTION, ISOLATION, AND PURIFICATION
23.2.1. Introduction: Two methods, refluxing and distillation, have already been discussed in section 22.3.6.i. FIG. 22.3 shows the equipment used.
Refluxing is used when volatile reagents must be heated together for any length of time. Distillation is used when a volatile product is formed by reaction between relatively non-volatile reagents. The case discussed in section 22.3.6.i. is a somewhat special case, but the principles still apply.
23.2.2. Distillation
Distillation may also be used to separate a volatile product from non-volatile impurities at the end of a reaction. The method is refined by:
i) Using a water bath to heat the mixture. For mixtures which boil at a higher temperature, an oil bath with thermometer should be used.
ii) Putting a few pieces of unglazed porcelain in the mixture to ensure steady boiling (anti-bumping granules).
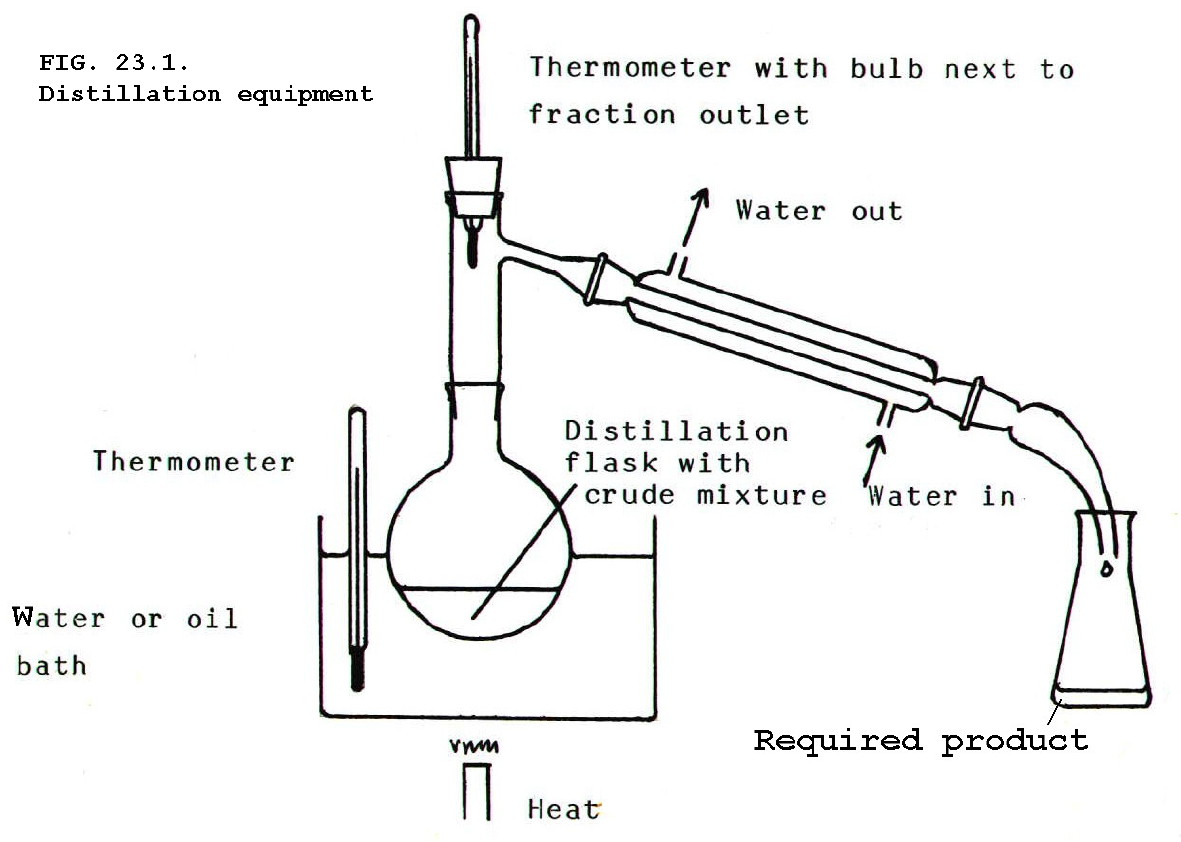
iii) Measuring the temperature at which the distillate comes over by using the equipment in FIG 23.1. Note that the thermometer bulb is level with the outlet.
iv) The water jacket
condensers are used only when the mixture boils at below 140°C,
otherwise there is danger of cracking the glass.
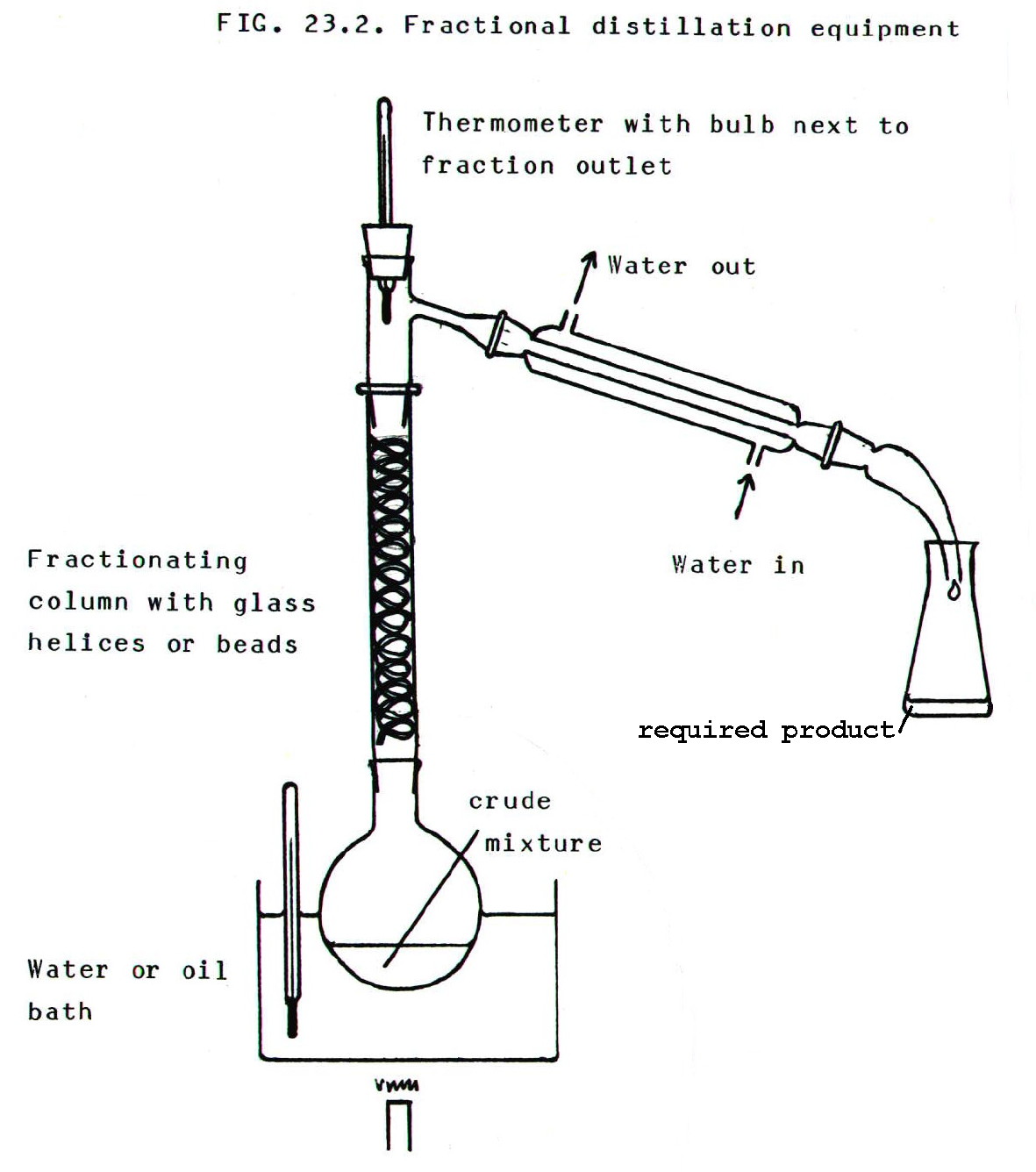
23.2.3. Fractional Distillation
For mixtures of volatile liquids a fractionating column must be used. See FIG 23.2. The fractionating column is shown without a water jacket, but for lower temperatures, a water jacket with reverse flow (FIG 23.1.) may be used.
The basis of the fractionating column is that temperature decreases up the column. As the vapour travels up the column, the higher boiling point components condense on the packing material and run back down the column. The condensed mixtures may revaporise, but the vapour becomes richer in the lowest boiling point component as it travels further up the column (section A3.3.3.).
The process is helped by the returning high boiling point components, which "wash" the rising vapour, and help high boiling point components to condense. Thus the more contact there is between rising vapour and returning liquid, the more effective is the separation. This is the role of the packing material.
The thermometer
measures the temperature of the distillate. Obviously the temperature
recorded should be equal to the boiling point of the pure low boiling
point liquid.
See also FIG. 26.1. for an industrial example of fractional
distillation.
23.2.4. Steam distillation
If the required product
in a mixture is volatile and the impurities are not, steam distillation
can be used.
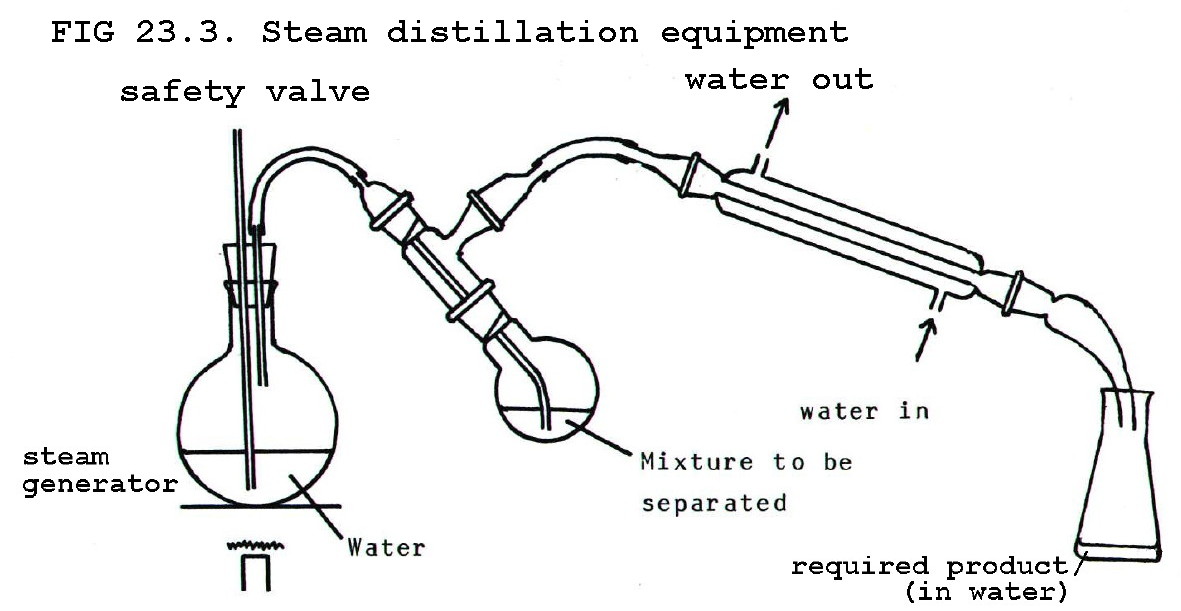
The product is obtained in a mixture with water. If it is a solid it
can be removed from the water by filtration. If it is a liquid, solvent
extraction may be used.
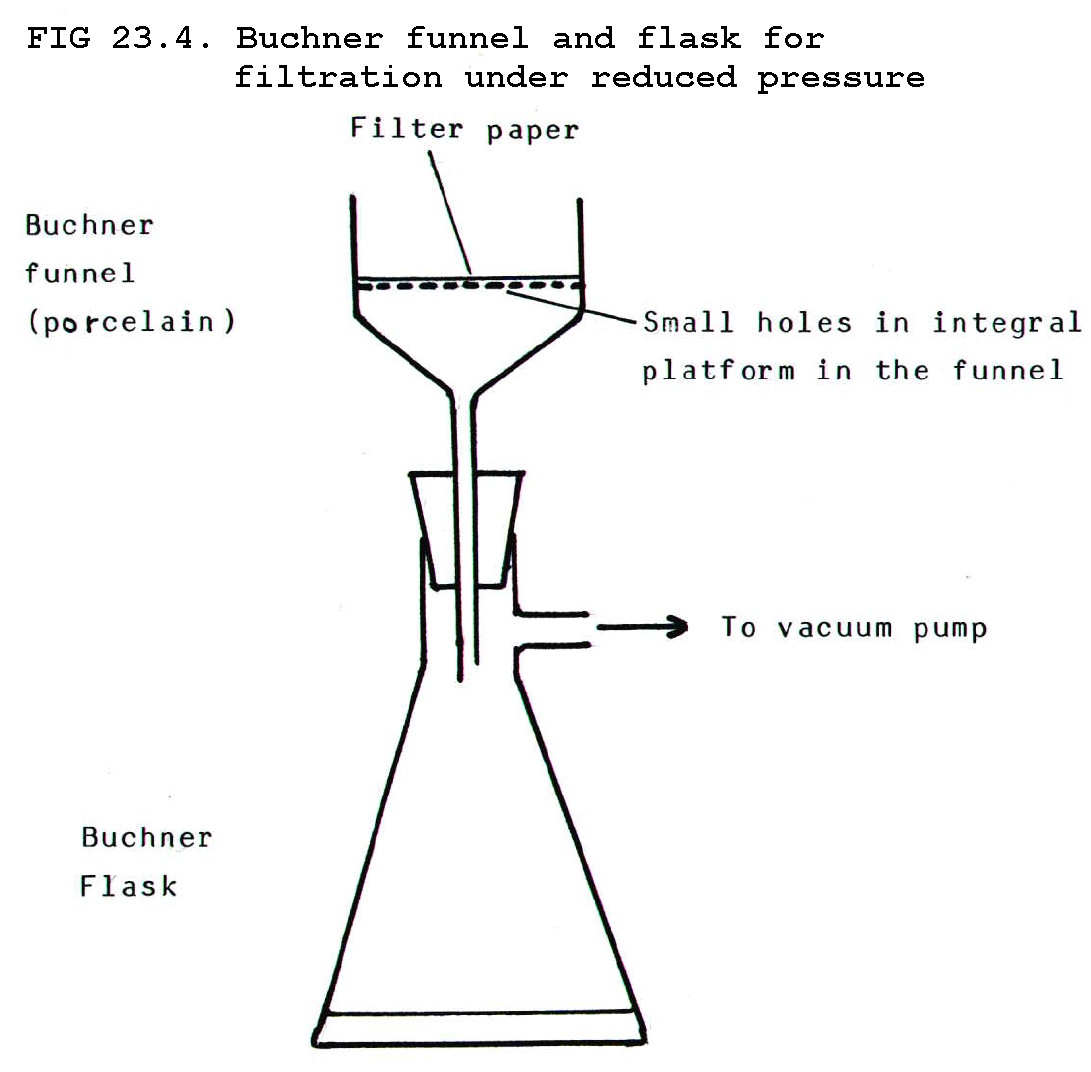
Note that filtration of
organic substances must often be carried out under reduced pressure in
order to speed the process. The apparatus used is a Buchner funnel and
flask (FIG 23.4).
23.2.5. Solvent Extraction (section A3.4.)
This method is often the first used after reaction. Laboratory preparations often produce a solution or suspension of the required product in water. Repeated solvent extraction will transfer the product into an organic solvent. The most commonly used organic solvent is ethoxyethane (diethyl ether) because:
i) Many organic compounds are soluble in ethoxyethane.
ii) It is insoluble in water.
iii) It is largely unreactive and therefore unlikely to react with the required product.
iv) It is easily removed from the compound because it boils at 35°C.
The mixture is shaken
with ethoxyethane in a pear-shaped funnel (FIG 23.5). Because ether is
volatile, the stopper must be released from time to time to let off the
pressure. The layers are allowed to separate and the ether layer is run
off through the tap.
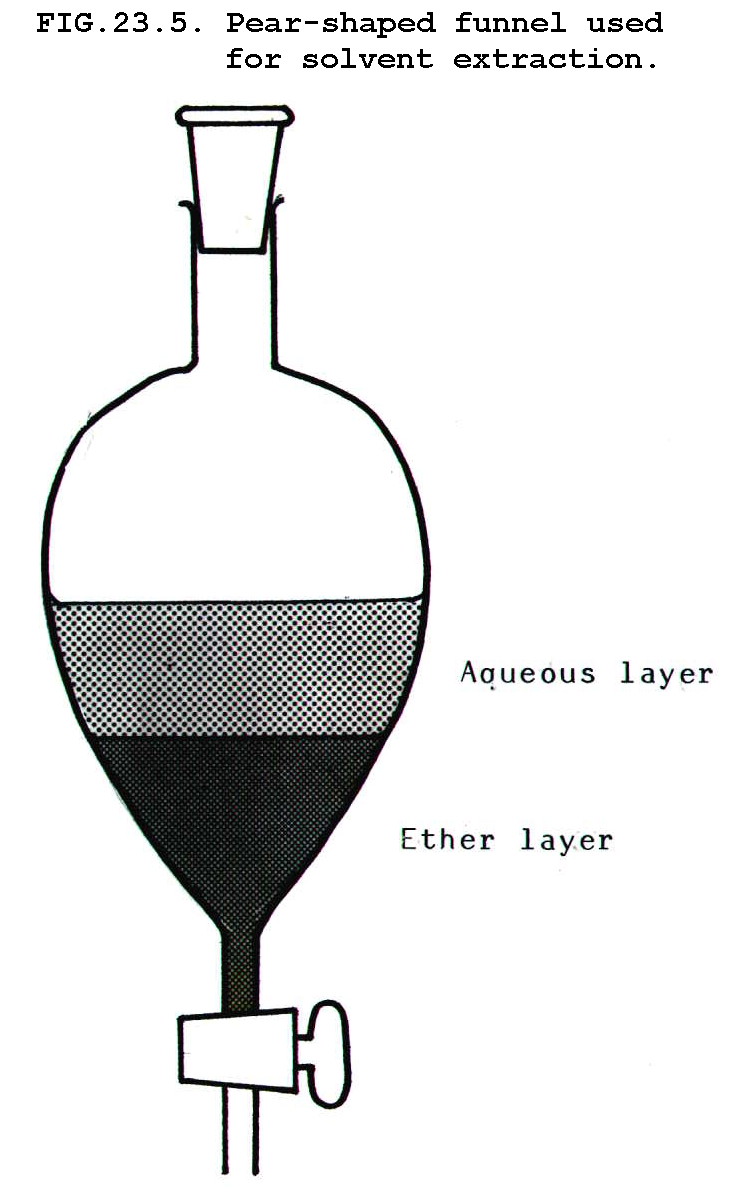
To encourage the product out of aqueous solution and into organic
solution, the aqueous solution is often saturated with salt. This
"salting out" is especially useful when the organic product is
particularly soluble in water.
The process is repeated on the remaining aqueous layer, maybe several times.
The ether extracts are then pooled and any remaining water is removed using one of the following drying agents:
i) Calcium sulphate(VI). Most universally useful drying agent.
ii) Anhydrous magnesium sulphate(VI). Can be used for almost any drying operation.
iii) Anhydrous sodium sulphate(VI). Can be used with almost anything, but it is not as fast as magnesium sulphate(VI).
iv) Calcium chloride. Not used for alcohols, phenols or amines because it forms addition compounds with them. Nor can it be used to dry carboxylic acids because calcium chloride always contains some calcium hydroxide which would react with the acid.
v) Potassium hydroxide. Often used for drying amines. Cannot be used for drying haloalkanes, phenols, acids or esters, because it reacts with them.
vi) Sodium wire. Quick
but reactive. Used mainly for drying hydrocarbons or ethers, but cannot
be used for anything that reacts with sodium or with alkali. Nor can it
be used to dry readily reduced compounds owing to the following
reaction with the water: 2Na + 2H2  2NaOH + H2!
2NaOH + H2!
23.2.6. Crystallisation
For this method a solvent must be found which dissolves the required compound when hot, but which dissolves it far less when cold. (*Or see below)
 The mixture is
dissolved in hot solvent,
The mixture is
dissolved in hot solvent,
 Filtered to remove
insoluble impurities,
Filtered to remove
insoluble impurities,
 cooled,
cooled,
 filtered again to
remove impurities which remain in solution,
filtered again to
remove impurities which remain in solution,
 washed,
washed,
 and dried.
and dried.
The process may be repeated until pure product is obtained (as indicated by a sharp melting point, for example).
*Alternatively, it may be the required compound which stays in solution, and more impurities which come out of solution when the solution is cooled.
23.2.7. Chromatography (section A3.4.)
The most common form of
chromatography used for separating reasonable quantities of substance
is column chromatography (FIG 23.6.). It is a frequently used method in
biochemistry. Thin layer, or paper chromatography is useful only for
analytical purposes.
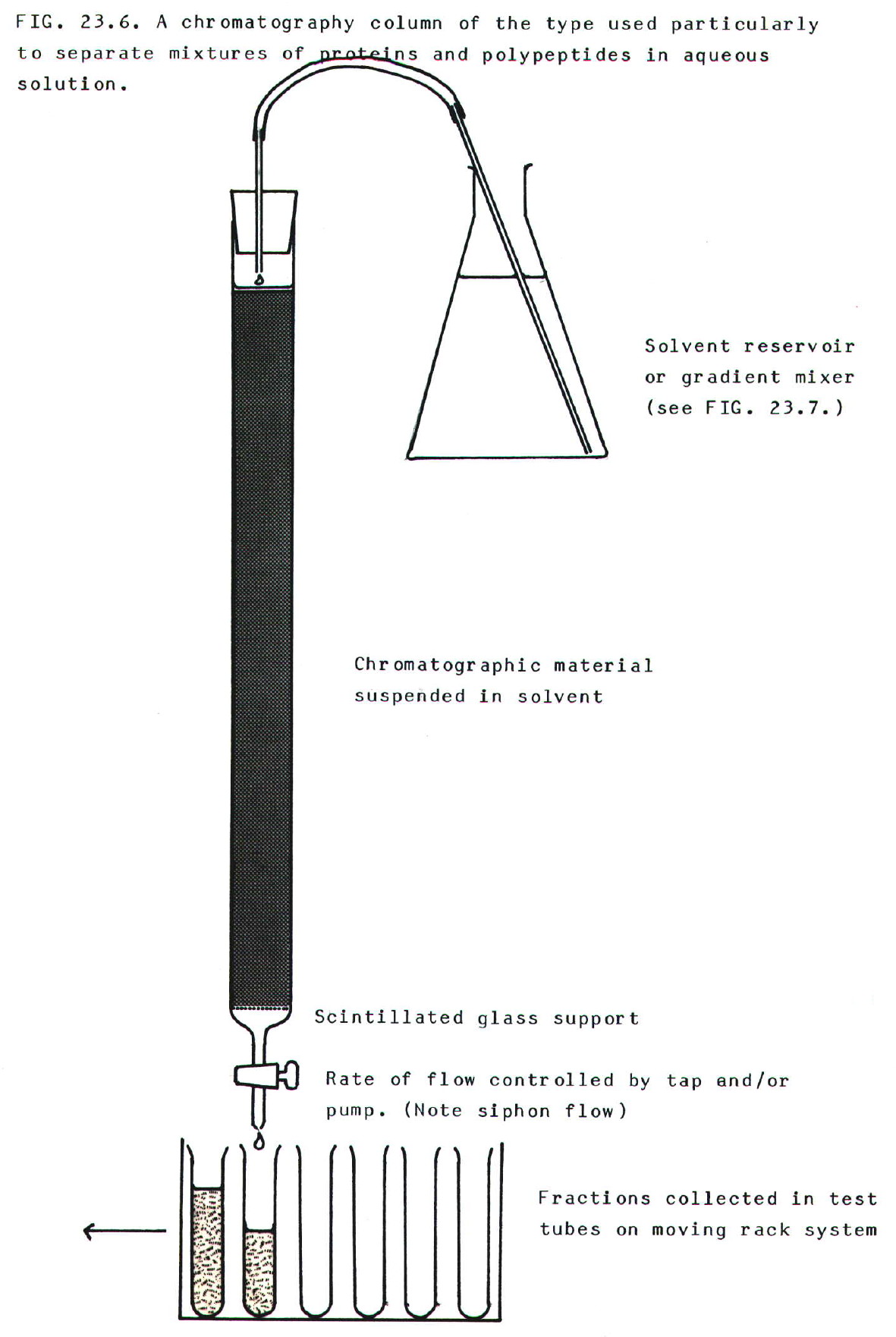
i) Adsorption or affinity chromatography: The
separation often relies on the fact that different substances bind to
the packing material in the column with different strengths. Those that
bind most strongly are washed through the column most slowly by the
flow of solvent. Thus different substances are collected in different
tubes on the moving rack (FIG.23.6.).
In biochemical applications, adsorption materials can be very specific. For example a particular enzyme can be separated from a mixture of proteins. The packing material may in this case be an enzyme substrate which has been bound to the surface of polysaccharide gel (in bead form). Since enzyme active sites are very specific for their particular substrates, purification can be near to 100%.
However, to remove a protein from such a column it may be necessary to wash with a different pH or different ionic strength of solution. Protein structure and enzyme activity are very sensitive to pH and ionic strength (section 25.2.5.).
ii) Gel filtration: The gels (eg sepharose or cellulose in biochemical applications) used to carry the substrate molecules are themselves used for a differrent type of chromatography known as gel filtration. The polysaccharide chains can be chemically cross-linked. Small molecules can then enter the beads of gel, but large molecules cannot, so they pass down the column more quickly. Moreover, the "mesh of the seive" can be determined by controlling the degree of cross-linking.
iii) Ion exchange chromatography: This relies on the fact that aqueous proteins (for example) have charged groups on their surfaces. Separation depends on the different degrees to which different protein ions bind to charged groups on the chromatographic material. The chromatographic material is often polysaccharide gel beads like those used in gel filtration.
Thus the protein (or other) ions exchange with the ions normally associated with the gel-surface groups. The stronger they bind, the more slowly they are eluted from the column.
Ion exchange resins, also in bead form, are used to separate mixtures of amino acids.
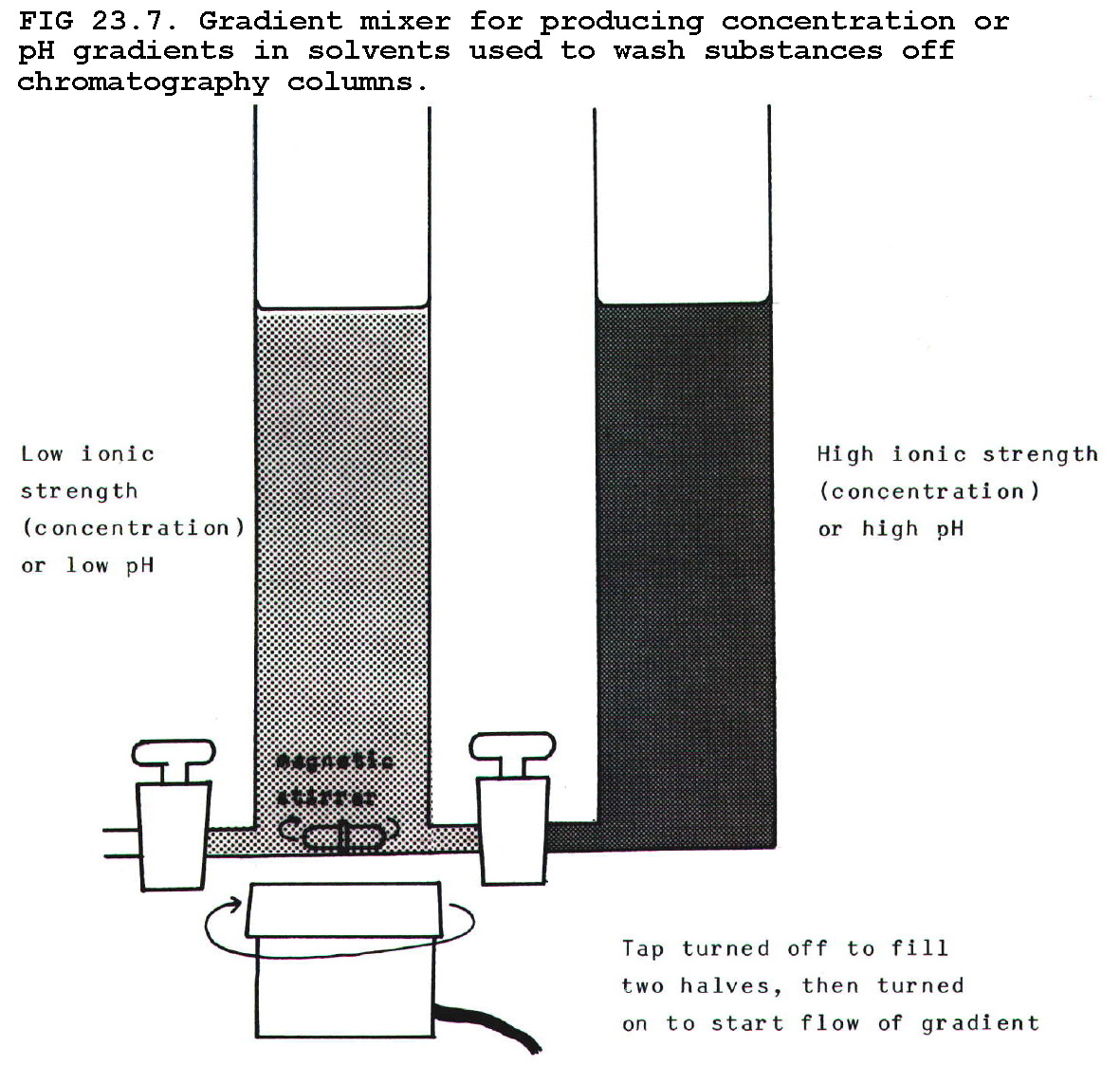
iv) Gradients:
In all three methods above, ionic strength and pH are important in
determining the separation, particularly because they affect the shape
and degree of denaturation of the protein chain. Resolution can be
increased by washing off proteins which remain stuck to a column, using
a concentration or pH gradient.
v) Partition chromatography: This relies on
the fact that the packing material may hold one solvent (often water)
and the mixture to be separated is dissolved in a different solvent. In
this case, different substances in the mixture may dissolve to
different degrees in the two solvents (partioning). Substances which
are most soluble in the solvent held by the packing material will pass
down the column most slowly. This is not such a common method in
biochemical techniques.
vi) Gas-liquid chromatography: In this case a gaseous mixture is passed down a column packed with an inert substance (e.g. celite, a form of calcium carbonate) impregnated with a non-volatile solvent (e.g. silicone oil). The mixture is washed down the column with an inert gas (e.g. nitrogen).
Different substances dissolve in the solvent to differing degrees. Those that are most soluble pass down the column most slowly.
Note that liquid or even solid mixtures can be separated in this way if they are volatile. The mixture is heated to vaporise it and passed down a column in a thermostatically heated oven.
When the emerging carrier gas contains one of the gases in the mixture, its thermal conductivity changes, and this is used as a detection method.
vii) Other detection methods: Thermal conductivity is not an appropriate detection method for the earlier types of chromatography discussed above. Moreover, it may be difficult to determine which tubes contain the substanec required if there is no distinctive separating feature such as colour. However, when there is no visible colour, it may still be possible to detect UV absorption using a spectrometer - proteins, for example, absorb at characteristic wavelengths.
Alternatively, a chemical substance may fluoresce under UV. Otherwise, it may be necessary to evaporate small samples from each tube, and test residues.
viii) Some final theory: The packing material or held solvent in chromatography columns is known as the stationary phase. The mixture passing down the column is the mobile phase. Brilliant!
In a given set of conditions, the rate at which substances pass down chromatography columns is characteristic of the substance, and it may be represented by the substance's Rf value:
..............Rf
..............=...............
Distance travelled by the substance
.................................................Distance
travelled by the solvent front
23.3. SUMMARY OF PREPARATIVE REACTIONS (Cross references).
For each class of compound in this section, a series of clues is followed by a list of cross-references. The clues are designed to help you work out reactions which could be used to prepare the class of compound in question. The cross- references refer to relevant reactions discussed in earlier sections of the book. If you need the cross-references, it will be a useful excercise to decide which clues refer to which cross-references (question 2, section 23.5.).
23.3.1. Alkanes:
Clue: With all that hydrogen around, reduction is going to be a key reaction. What organic compounds can be reduced to alkanes, and how?
Cross-references:
21.3.4. (also applies to alkynes)
Table 21.5 and note following
21.7.11.ii&iii.
22.3.10.ii.
23.3.2. Alkenes:
Clue: What is the reaction which produces double bonds and what classes of compound undergo that reaction?
Cross-references:
21.7.3.
22.1.5. and FIG. 22.1.
Elimination in alcohols may also be brought about by passing the alcohol vapour over heated alumina. The ease of dehydration by this method follows the pattern discussed in 22.1.5: primary 350°C, secondary 250°C, tertiary 150°C.
23.3.3. Alkynes:
Clue: If elimination introduces a double bond, double elimination introduces a triple bond, eg from CH3CHBr.CH2Br, or from CH3CHCl2, for example. Neither of these substances is particularly common in the laboratory, but they are easily prepared from common laboratory reagents. How can two halogen atoms be introduced into a molecule either on adjacent carbon atoms or on the same carbon atom?
Cross-references:
Table 21.2.
22.2.6.v.
In other words, it is easy to prepare alkynes from alkenes and from carbonyl compounds.
23.3.4. Benzene:
Clues and cross-references: Creating a benzene ring is obviously not easy, so any laboratory preparation is probably a bit of a cheat eg decarboxylation of sodium benzoate (section 22.3.10.ii.). Alternatively, elimination from 1,3,5-tribromocyclohexane can be a final step in several long-winded procedures.
In short, benzene rings are not easily synthesised and are obtained from naturally occurring sources. More relevant is how various benzene derivatives are made. Obviously, electrophilic substitution into the ring is a key starting point. Further reactions are then important:
Derivatives: cross-references: Table 21.3. especially nitration and acylation. Both these reactions take on special significance when followed by reduction. The methods can NO2, RCO, NH2 (22.7), and R (preferably via acylation table 21.3)
Table 22.6. OH, F, Cl, Br, I, CN, (NB CN COOH or CH2NH2, section 22.6), and H. Further derivatives by reaction of these side chains.
23.3.5. Haloalkanes:
Clues: The type of reasoning in section 23.1 should help you work out at least one method of preparing haloalkanes. Applied more generally you should be able to predict at least three techniques.
Think of three types of reaction which introduce one (or two) halogen atoms into a carbon chain, and you have the three main methods for synthesising haloalkanes. Different angle: what type(s) of reactant is a halogen molecule, and what type of reactant is a halide ion?
Cross-references:
21.1.1. and Table 21.1.
21.3.1. and Table 21.2.
22.2.1./22.1.3.i.
(Also 22.2.6.v.
23.3.4.)
23.3.6. Alcohols
Clues: One method has already been deduced in section 23.1. If you have just worked through section 22.3.5. you should be able to work out at least one other method.
Cross-references:
21.3.1., Table 21.2. and FIG. 22.1.
21.7.2. and Table 21.5
22.3.4. and 22.3.6. Note phenolic esters give phenol.
22.4.5.i.
22.2.6.ii. and FIG 22.2.
From Grignard reagents (see section 23.4.)
Also 23.3.4.
23.3.7. Aldehydes and ketones (carbonyls)
Clue: One method should spring to mind as representing the place of carbonyls in the, by now over-familiar, alcohol carbonyl carboxylic acid redox series.
Cross-references:
21.3.5.i.c.
22.3.6.v. (Re-read this section carefully and don't jump to conclusions)
22.1.12.i and FIG 22.2.
Hydrolysis of gem-dihalides, ie. halides with both halogens on the same carbon atom. The resultant gem-dihydroxy-compounds are unstable and lose water immediately to form carbonyl compounds:
...............................................OH
............................................../
CH3CHCl2.......... ..........
CH3CH..........
..........
CH3CH.......... ..........
CH3CHO....
+.... H2O
..........
CH3CHO....
+.... H2O
..............................................\
...............................................OH
CH3CCl2CH3....... .......
CH3C(OH)2CH3.......
.......
CH3C(OH)2CH3....... .......
CH3CO.CH3.... +....
H2O
.......
CH3CO.CH3.... +....
H2O
As a matter of interest, one of the few stable gem-dihydroxy compounds is 2,2,2-trichloroethanediol (chloral hydrate). There is a significant amount of this in equilibrium with the carbonyl form, trichloroethanal (chloral).
CCl3CHO.... +....
H2O.......... ..........
CCl3CH(OH)2.
..........
CCl3CH(OH)2.
Also 23.3.4.
23.3.8. Carboxylic acids:
Clue: What redox series should immediately spring to mind?
Cross-references:
22.1.12. and FIG. 22.2.
22.2.6.i.
22.6.i.
From Grignard reagents (see section 23.4.)
23.3.4.
23.3.9. Carboxylic acid derivatives:
Clue: So, how are you going to get that derivative group in there?
Cross-references:
Acid chlorides 22.3.4. and 22.3.6.vi.
Acid anhydrides 22.3.4. and 22.3.6.iv.
Esters 22.3.4. and 22.3.6.ii.
Amides 22.3.4. and 22.3.6.iii.
(Also 23.3.4.)
23.3.10. Amines:
"Clues": If you are unable to predict one method by now, you might as well be told straight: nucleophilic substitution using ammonia as nucleophile to replace halogen atoms in haloalkanes (21.7.2. and Table 21.5). In fairness, this is not a very controllable reaction, as discussed in section 22.4.4.i.
So with all that hydrogen around, what other type of reaction do you think might be useful?
Cross-references:
22.3.6.v.
22.3.11.i.
22.6.ii.
22.7.
23.3.4.
23.3.11. Nitriles:
Clues: What kind of reactant is a -CN ion? What other compounds have a nitrogen atom attached to a carbon atom, and which of them might be a useful starting material?
Cross-references:
21.7.2. and Table *21.5.
22.3.11.ii.
23.3.4
Note that to introduce an iso-nitrile group, -NC, the moist silver salt provides a source of iso-nitrile ions as nucleophiles.
23.3.12. Nitro-compounds:
Clues: Just as we are getting into the swing of things we finish on a downer. The nitrate(III) ion is not a particularly useful nucleophile for what we want. So how else can we get a nitro group into an alkyl chain? Getting one into a benzene ring is easy enough - remember? But, what difficult method do we know for introducing groups into alkyl chains?
Cross-references:
Table 21.5.
21.2.1. and Table 21.1.
21.6.1. and Table 21.3. (Already referred to in 23.3.4.)
23.4. Grignard reagents.
23.4.1. Introduction: The preparation of Grignard reagents has already been discussed in the note following Table 21.5. Their usefulness was also mentioned, and various examples have been referred to in the table itself, and in section 23.3. above.
The availability from Grignard reagents of alkyl chains, which are able to act as nucleophiles, is about as extraordianry as the availability from diazonium compounds of benzene carbonium ions, which are able to undergo nucleophilic attack. The possibilities are almost as endless (sections 23.4.3. to 23.4.9.):
Note that Grignards are not usually isolated, but are used for the further reactions in the dry ethereal solution. The effect of water is shown in section 23.4.2. However, the initial products of reactions with Grignards must often be hydrolysed to give the desired product.
23.4.2. With haloalkanes nucleophilic substitution gives alkanes:
R´MgX.... +....
RX....... .......
R´R.... +.... MgX2
.......
R´R.... +.... MgX2
23.4.3. With water Grignards give alkanes:
R´MgX.... +....
H2O....... .......
R´H.... +.... Mg(OH)X
.......
R´H.... +.... Mg(OH)X
23.4.4. With appropriate carbonyls nucleophilic addition gives primary, secondary, or tertiary alcohols (Table 22.1.). E.g.:
R´MgX.... +....
RCHO....... .......
R´RCHOMgX
.......
R´RCHOMgX
....................hydrolysis
R´RCHOMgX....... .......
R´RCHOH.... +.... MgXCl
.......
R´RCHOH.... +.... MgXCl
...................e.g.
dil HCl
23.4.5. With epoxides nucleophilic addition produces primary alcohols:
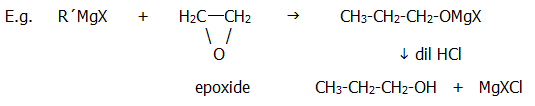
The epoxides are produced when alkenes react with, for example, bromine water (Table 21.2.). The halohydrins (with halogen and OH groups on adjacent carbon atoms) readily lose halogen acids to form epoxides such as the epoxyethane in the above equation.
23.4.6. With carbon dioxide nucleophilic addition produces carboxylates:
R´MgX.. +..
O=C=O....... .......
R´COOMgX
.......
R´COOMgX
................................................ 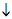 dil HCl
dil HCl
....................................... R´COOH.. +.. MgXCl
This reaction provides the basis of an alternative method for introducing a carboxyl group into a benzene ring (cf 23.3.4.):
23.4.8. Oxidation of a Grignard to the corresponding alcohol is not a particularly helpful reaction, since hydrolysis of the original haloalkane produces the same alcohol more easily.
23.4.9. Lengthening carbon chains (ascending homologous series) is one particularly useful function of Grignard reactions. The reaction with carbon dioxide lengthens it by one carbon atom, the reaction with epoxyethane by two, and the reaction with haloalkanes by virtually any number. The epoxide reaction can also potentially increase the chain length by any number of carbon atoms depending on the choice of epoxide.
A typical useful series of reactions might be:
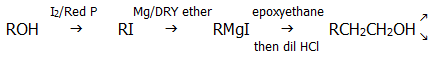
23.5. QUESTIONS
1) For each of the major classes of compound covered in sections 23.3.1. to 23.3.12. choose one named example and describe in reasonable detail how you would prepare a sufficiently pure sample.
2) In each of the sections 23.3.1. to 23.3.12. a series of clues is followed by a series of cross-references to help you work out the main methods of laboratory preparation. For each section explain which clues refer to which cross-references.
Eg. In section 23.3.10, the clue in paragraph 2 is trying to make you think of reduction reactions. Cross-references 22.3.6.v and 22.6.ii. and 22.7 refer to simple cases of reduction. Cross-reference 22.3.11.i. refers to a reaction which is a more complex reduction, but which is not primarily thought of as such. This would be your starting point for answering the question with respect to section 23.3.10.
3) Using reactions referred to in section 23.3.4. as your starting point, how could you prepare benzenecarbaldehyde (ArCHO) from benzene in the laboratory. What further possible method is there?
Unless otherwise stated, all materials in this web version of chapter
23 are © 2007 Adrian Faiers MA (Oxon) MCIPR

What 's the connection between a dozen eggs and
a garden mole?

Answer: Not a lot, really, but see Chapter 1