Recommended by:

Top 20 UK science resources,
The Tutor Website
Recommended by:

Rated:

2010
Listed on the science, engineering and technology section of

'providing you with access to the very best Web resources for education and research, evaluated and selected by a network of subject specialists.'
(Please note that intute closed in July 2011)
Section 3: Organic
CHAPTER 24: ISOMERISM
NB This chapter has now been
updated to improve browser compatibility.
Please
use the 'send email' link at the top right hand corner of this page to
report any problems.
24.1. INTRODUCTION
Isomers are compounds with the same molecular formulae, but different arrangements of atoms within the molecule, and therefore different physical and/or chemical properties.
There are several types
of isomer; some are examples of more general types, and the picture can
become confusing. FIG 24.1. clarifies the picture by describing the
interrelationships amongst the main types of organic isomer.
(Click on diagram to
enlarge on a new web page)
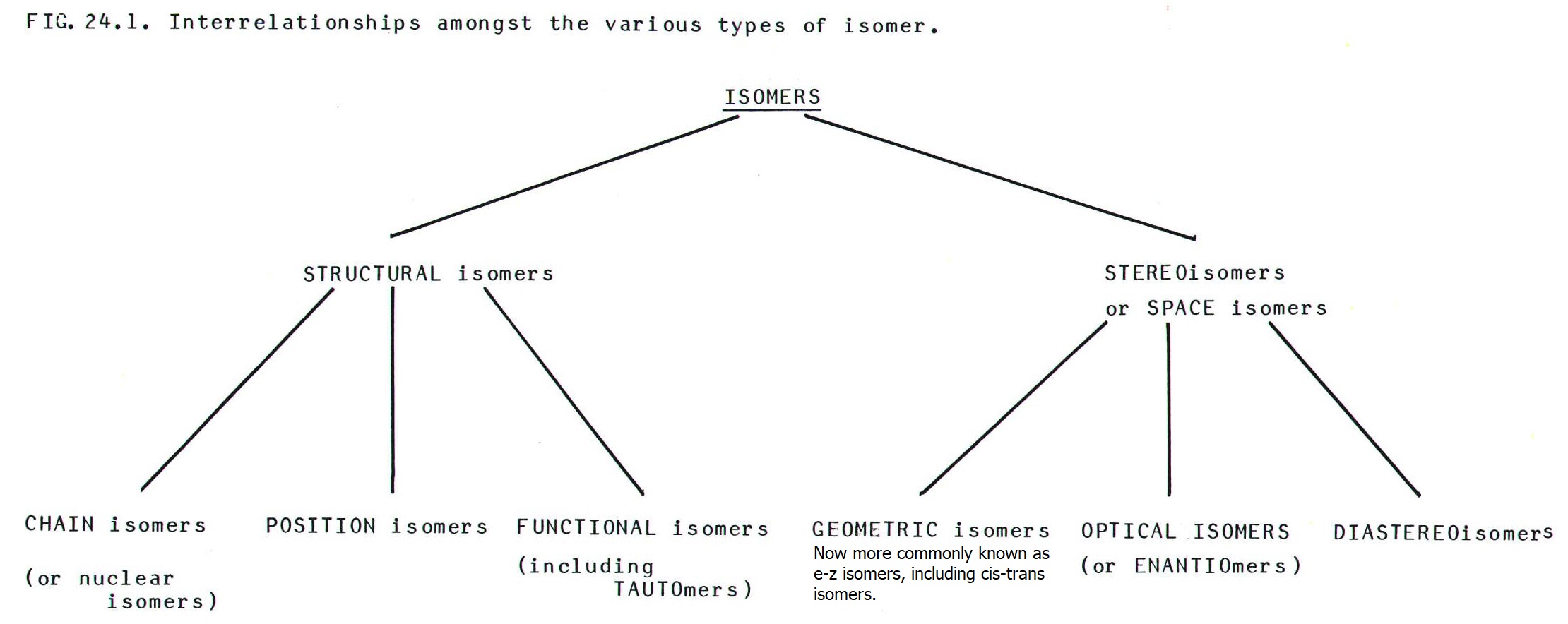
24.2. STRUCTURAL ISOMERS
24.2.1. Structural isomers differ in the order in which the atoms are joined together. There are three types of structural isomer: chain, position, and functional.
24.2.2. Chain isomers differ in the arrangement of carbon atoms in the molecule.
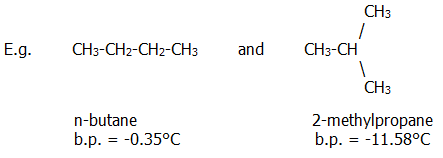
Branching in 2-methylpropane prevents the molecules from packing so closely together, thus reducing the effectiveness of van der Waals bonding. This, in turn, reduces its melting and boiling points.
24.2.3. Position isomers differ in the position of a particular atom (or group of atoms) on a carbon chain or ring.
E.g. .......CH3CH2CH2OH .......and ....... CH3CHOHCH3
..............propan-1-ol ..........................
propan-2-ol
..............b.p.
= 97.35°C .....................
b.p. = 82.65°C
24.2.4. Functional isomers differ in the arrangement of atoms, such that they have different functional groups.
E.g. ......CH3CH2
................
CH3
..............\ ........................... \
..... .........C=O .......
and ....... C=O
............../ ........................... /
............H .......................... CH3
............propanal ................ propanone
............b.p.
= 48.15°C ....... b.p.
= 56.44°C
Note that aldehydes and ketones also differ significantly in their chemical properties.
24.3. STEREOISOMERS
24.3.1. Stereoisomers have the same atoms and groups of atoms bonded together in the same order; they differ only in the spatial orientation of the atoms and groups. There are three types of stereoisomer: geometric, optical, and diastereoisomers.
24.3.2. Geometric isomers differ in the arrangements of dissimilar atoms/groups attached to two atoms which are joined by a double bond or which form part of certain ring structures.
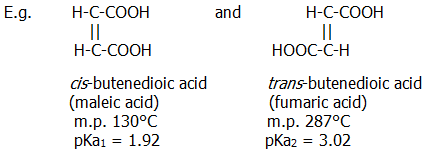
There is also another interesting chemical difference between the cis and trans acids. Owing to the conducive positioning of the carboxyl groups in cis-butenedioic acid, the acid readily forms an internal anhydride, whereas the trans acid does not.
Cis-trans isomersim is a special case of e-z isomerism. Exam boards these days tend not to accept the term geometric isomersim to cover this type of isomersim across a double bond and they demand use of the term e-z isomerism.
The e-z nomenclature places the groups attached across a double bond in order of 'priority' according to a particular *set of rules. When the two highest priority groups are on the same side of the double bond the isomer is the z isomer. The e isomer is the isomer with the two highest priority groups on opposite sides (e = enemies, is a good way to remember which is which). *See http://en.wikipedia.org/wiki/E-Z_notation for the set of rules.
24.3.3. Optical isomers, or enantiomers, differ in the arrangement of atoms, such that the two forms are non-superimposable asymmetric mirror images of each other.
The simplest source of
asymmetry is an asymmetric carbon atom i.e. one with four different
groups attached to it. E.g. as in 2-hydroxypropanoic acid shown in FIG
24.2.
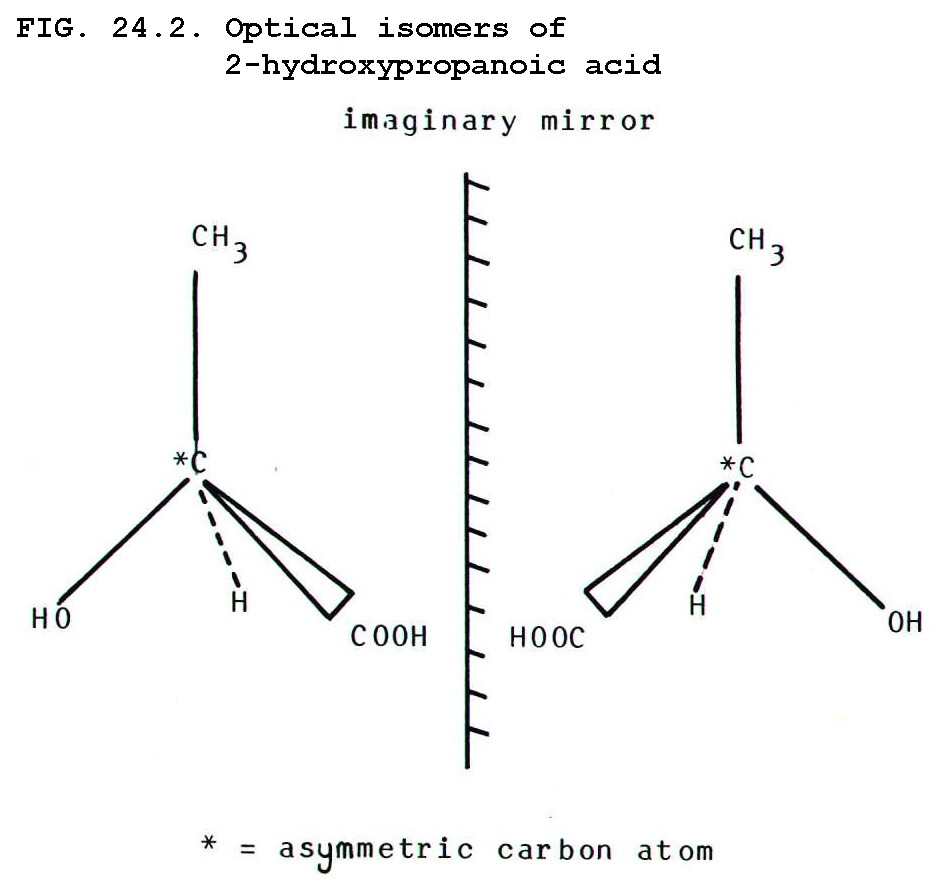
In the diagram, bonds which should be imagined as in the plane of the
paper are shown as solid lines. Those which should be imagined as
coming out of the paper are shown as wedges, and those which should be
imagined as going into the paper are shown as dotted lines. Sometimes
it is more convenient to represent bonds going into the paper as wedges
with the sharp end pointing into the paper (see FIG. 24.4.)
To check the non-superimposability of the two molecules, imagine picking up the enantiomer on the left by its CH3 group. Then imagine turning the molecule in a clockwise direction (viewed from above) until its COOH group is superimposed on the COOH group of the molecule on the right. Where is the OH group of the first molecule now? It is lying over the H atom of the second molecule, showing that the two enantiomers cannot be superimposed.
If you find this hard to imagine, build yourself simple models. Use pieces of plasticine or ®Blu-Tack as the central asymmetric carbon atoms and matchsticks to represent the four bonded groups in each optical isomer. Then use felt pens to give each type of group (represented by matchsticks) a different colour.
Almost the only difference in properties between optical isomers of a compound, is the effect they have on plane polarised light passed through their solutions or crystals. Both enantiomers rotate the plane of polarisation, and they both rotate it by the same angle, but one form rotates it in one direction and the other optical isomer rotates it in the opposite direction.
Plane polarised light
which has passed through a (+)-, or dextrorotatory, enantiomer is
rotated to the right (clockwise) as seen with the light coming towards
the observer. Plane polarised light which has passed through the (-)- ,
or laevorotatory, enantiomer appears rotated to the left.
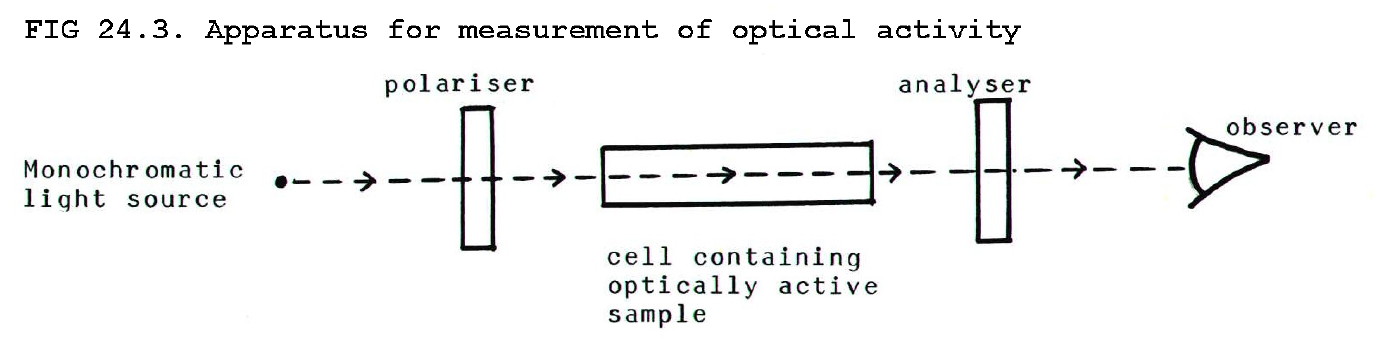
The right- or left-handedness of optical isomers is referred to as
their chirality. Mixtures which contain both
optical isomers of a compound in equal quantities show no optical
activity because the effect of one isomer cancels the effect of the
other. Such mixtures are known as racemic mixtures.
24.3.4. Diastereoisomers differ in their arrangement of atoms, such that the molecule is not superimposable on the other forms, but is not a mirror image of the other forms, does have a plane of symmetry, and shows no optical activity.
Optical isomers do not have a plane of symmetry. If a plane of symmetry can be drawn through a molecule, it will not show optical activity. Such a plane (imaginary) divides the molecule into two symmetrical halves.
The simplest diastereoisomers have two asymmetric carbon atoms. When two (+) centres are joined to each other, the molecule is a (+) enantiomer and rotates plane polarised light to the right. When two (-) centres are joined to each other the molecule is a (-) enantiomer and rotates plane polarised light to the left. When a (+) centre is joined to a (-) centre the molecule is a (±) diastereoisomer and shows no optical activity.
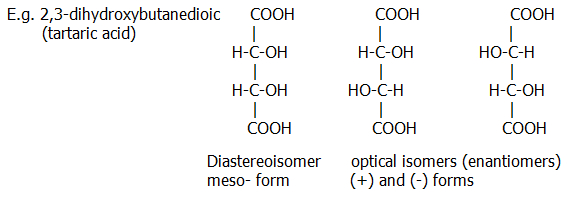
The projection shown in this diagram is known as the Fischer projection. Groups which should be imagined as in the plane of the paper or behind the paper (usually the main carbon chain) are projected vertically. Groups which should be imagined as in front of the paper are projected horizontally.
Diastereoisomers differ quite markedly in their properties from the optical isomers, which do not differ significantly from each other (apart from optical activity). Differences in solubility are common, and these can form the basis of methods for separating simple optical isomers.
E.g. To resolve a mixture of (+) and (-) 2-hydroxypropanoic acid (lactic acid = L).
l React with one enantiomer of an optically active base such as (+) quinine (Q)
l Separate the diastereoisomer (-)L(+)Q from the (+) enantiomer (+)L(+)Q by fractional crystallisation.
l Liberate the free acids by treatment with strong acids.
This is the best type of method. Two others are:
i) The crystals of enantiomers are often mirror images of each other and can be separated by hand-picking! Alternatively a solution of the two optical isomers can be seeded with crystals of one enantiomer. Only that particular isomer will grow on the seeding crystals.
ii) With some compounds, certain bacteria will consume only one enantiomer if grown in a solution containing both. Hopefully they will leave the one you want.
24.5. STEREOCHEMISTRY OF REACTIONS.
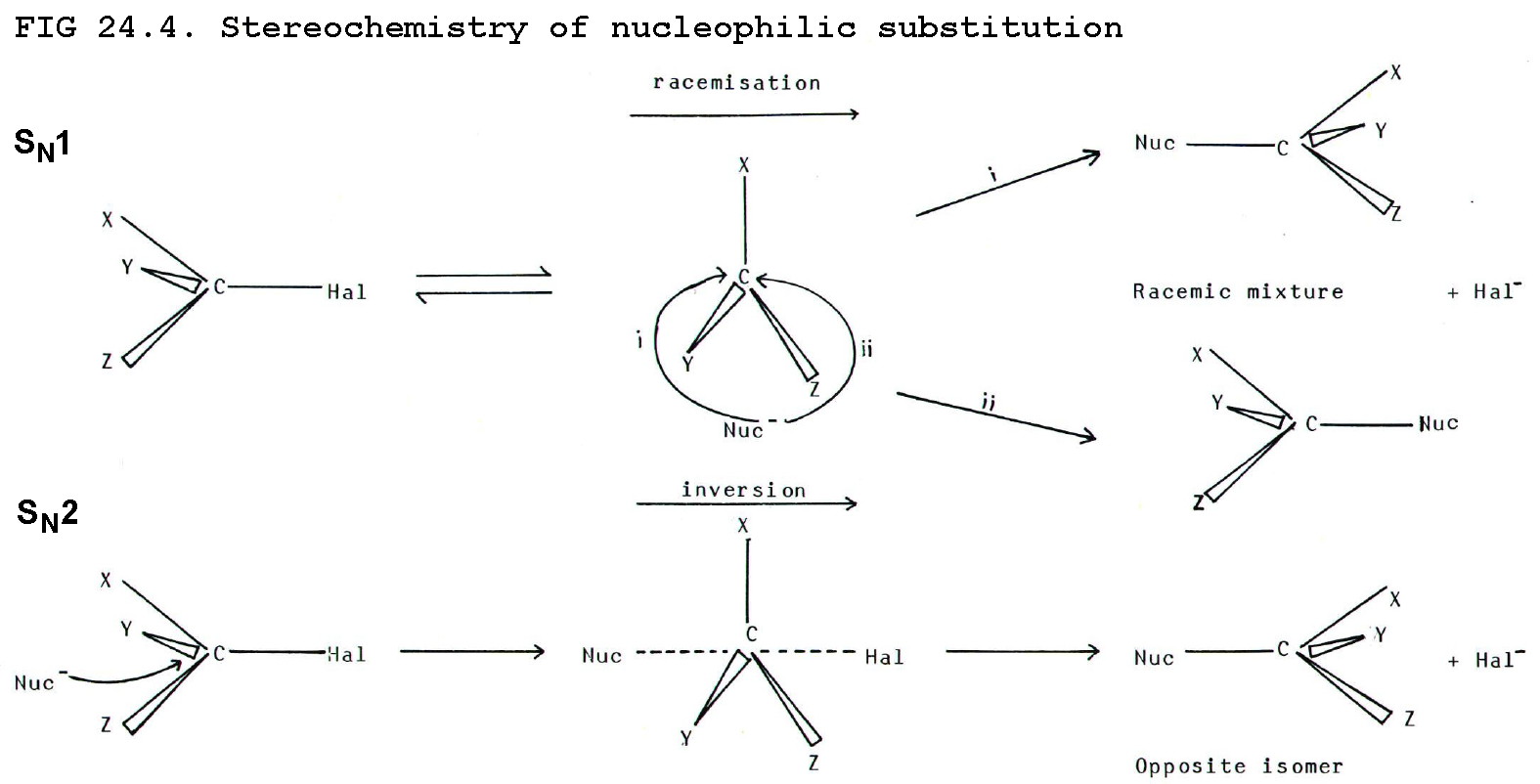
24.5.1. Some reactions produce one particular isomer. Others produce a racemic mixture. If the starting molecule is optically active, some reactions give a product in the same form, others invert the structure, and others bring about racemisation.
24.5.2. Nucleophilic
substitution is a particularly interesting example. The
stereochemistry of the reaction can distinguish between SN1
and SN2 mechanisms.
(Click on diagram to
enlarge on a new web page)
24.6. QUESTIONS
1) How useful is fractional distillation as a method for separating the pairs of isomers described in this chapter? Describe some other methods that could be used for separating the pairs.
2) Give the names and structures of position isomers of dibromobenzene.
3) Describe three chemical tests that you could use to distinguish between the two functional isomers discussed in section 24.2.4.
4) Show the mechanism of internal anhydride formation in cis-butenedioic acid.
5) Geometric isomerism is an example of structural isomerism which is, in turn, one of the two main classes of isomerism. Comment, explaining clearly the meaning of any term (relating to isomerism) which you use.
6) 2-hydroxypropanoic acid can be prepared in the laboratory from ethanal using "HCN" followed by hydrolysis. Will the product show optical activity? Explain, showing the stereochemistry of any relevant mechanisms.
7) 2-methylpropane differs from butane in the position of a methyl group on the carbon chain. 2-methylpropane and butane are therefore position isomers like propan-1-ol and propan-2-ol. Comment.
Unless otherwise stated, all materials in this web version of chapter
24 are © 2007 Adrian Faiers MA (Oxon) MCIPR

What 's the connection between a dozen eggs and
a garden mole?

Answer: Not a lot, really, but see Chapter 1