Recommended by:
Top
20 UK science resources,
The Tutor Website
Recommended by:

Rated:

2010
Listed on the science,
engineering
and technology section of 
'providing you with access
to the very best Web resources for education and research, evaluated
and selected by a network of subject specialists.'
(Please note that intute closed in July 2011)
Section 3: Organic
CHAPTER 26:
OCCURRENCE AND INDUSTRIAL RELEVANCE
OF SOME ORGANIC COMPOUNDS
NB This chapter has now been
updated to improve browser compatibility.
Please
use the 'send email' link at the top right hand corner of this page to
report any problems.
26.1. INTRODUCTION
In this chapter we shall look at the occurrence and industrial relevance of the main categories of organic compound. Some references to industrial processes have already been made in previous chapters, and those will be listed here under the appropriate classes of compound.
26.2. HYDROCARBONS
26.2.1. Occurrence of hydrocarbons: The main sources of hydrocarbons are:
i) Natural gas which is mainly methane but also contains other gaseous alkanes.
ii) Petroleum the composition of which varies from source to source. The components of petroleum are separated by fractional distillation and they include:
a) straight and
branched chain alkanes up to about 40 carbon atoms
b) napthenes (cyclic alkanes)
c) aromatic hydrocarbons
Alkenes are obtained from petroleum by cracking, a process which generally produces smaller, more useful, molecules.
Aromatic hydrocarbons are obtained from petroleum mainly by catalytic cracking of the higher fractions at around 650°C and by reforming the lower fractions.
iii) Coal/coal tar: Coal tar is obtained by destructive distillation of coal. The most volatile fraction from fractional distillation of coal tar contains simple aromatic hydrocarbons.
iv) Lime and coke: Ethyne is obtained from this source via calcium(II) dicarbide. The dicarbide is treated with cold water (section 21.4.2.iii.).
26.2.2. Fractional distillation of petroleum
This is carried out in
a fractionating column at an oil refinery, as shown in FIG. 26.1.
(Click on diagram to
enlarge on a new web page)
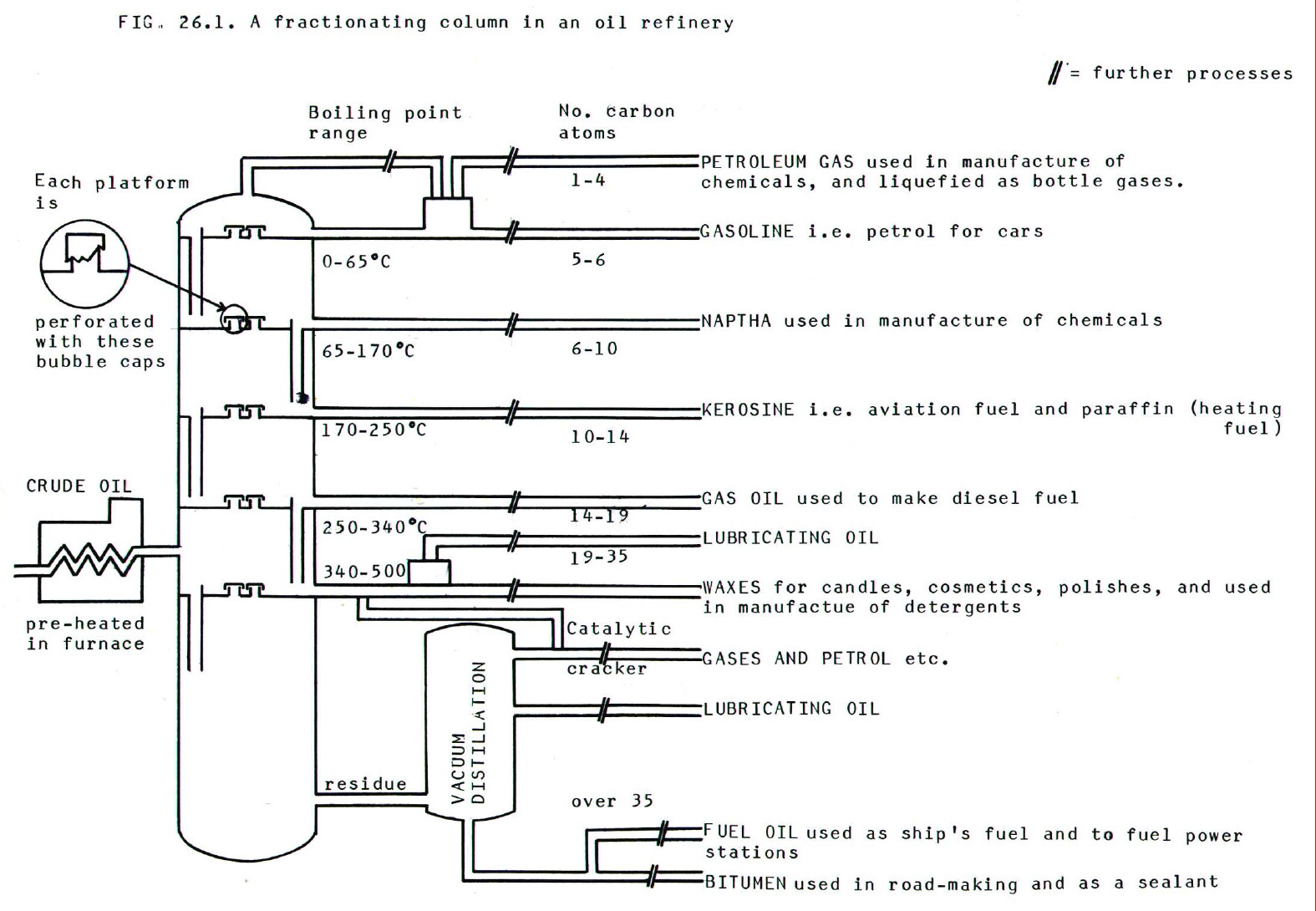
Two main factors affect the boiling point of alkanes:
- Chain length - the longer the chain, the greater the surface contact and thus the greater the number and total strength of Van der Waals forces making boiling point (and ,melting point) increase with increasing chain length;
- Amount of branching
-
branching prevents the chains from making such close contact, reducing
the number and strength of the Van der Waals forces between them and
hence reducing boiling (and melting) point.
26.2.3. Cracking
Temperatures range from 400 to 700°C, and catalysts such as oxides of aluminium, silicon, chromium, and thorium are used:
CxH2x+2 ......→......CyH2y+2...... +...... CzH2z...... (Where x = y + z)
alkane alkane................. alkene
(See also section 21.2.3.i.) The main alkenes obtained from cracking are ethene and propene, though one of the main roles of cracking is to generally produce the more volatile fractions. These are used as petrol (gasoline) and as starting materials in the petrochemical industry.
One of the alkanes which burns most efficiently in petrol is 2,2,4-trimethylpentane (iso-octane). A 100 octane fuel is one which burns as if it were 100% iso-octane. O on the scale corresponds to a fuel which burns as if it were 100% heptane, i.e. a straight chain alkane. Low octane fuels do not burn as efficiently. In particular they ignite too soon in the engine's cycle, an effect known as knocking, because of the sound it produces.
The octane value of a fuel can be increased by anti-knocking agents like tetraethyl lead, (C2H5)4Pb. It is also particularly important to remove sulphur impurities, which lower the octane value.
26.2.4. Reforming
The three main processes of reforming are:
i) Dehydrogenation
E.g. CH3-CH3 ........→....... CH2=CH2 + H2
ii) Isomerisation
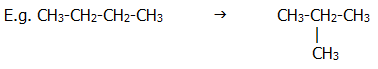
iii) Aromatisation
This process is carried out under pressure at about 500°C on the lower petroleum fractions. Catalysts such as platinum are used.
E.g. CH3CH2CH2CH2CH2CH3 .......→....... C6H6 ... + ... 4H2
CH3CH2CH2CH2CH2CH2CH3 .......→....... C6H5CH3 ... + ... 4H2
Methylbenzene and other benzene homolgues can themselves be catalytically reduced to benzene by hydrogen under pressure
E.g. C6H5CH3 ..... + ..... H2 .........→......... C6H6 ..... + ..... CH4
26.2.5. Further industrial relevance of hydrocarbons: Apart from the uses of hydrocarbons listed in FIG. 26.1, other references occur in previous chapters:
20.12.2. Manufacture of plastics, polymerisation
21.2.2. and Table 21.1. Manufacture of detergents
21.3.2. and table 21.2. Manufacture of ethanol
Table 21.3. Manufacture of styrene and hence polystyrene
21.6.8.ii. Manufacture of benzenecarbaldehyde
21.6.8.ii. Manufacture of varnishes
26.3. HALOGEN COMPOUNDS
Halogen compounds are made by halogenation of hydrocarbons using halogens or, in the case of alkenes, hydrogen halides as well as halogens. They are used as anaesthetics, insecticides, refrigerants (e.g. CCl2F2 which is also used as an aerosol propellant), solvents (e.g. 1,1,1, trichloroethane used in typewriter correction fluid), fire exstinguishers (section 21.7.11.i.), and in the manufacture of plastics (e.g. section 20.12.2.).
26.4. ALCOHOLS,
PHENOLS, AND ETHERS
26.4.1. The industrial manufacture of alcohols from alkenes has already been given in section 21.3.2. Another major method is fermentation used in the brewing industry:
..................enzymes in
yeast
C6H12O6
..............→..............
2C2H5OH ..... + ..... 2CO2
Alcohols also occur in nature, mainly as esters with carboxylic acids. Phenols are obtained from coal tar by extraction with alkali and also by autoxidation of 2-methyl-2-phenylpropane (cumene) which is obtained from petroleum.
26.4.2. Uses of alcohols include: • social drug (ethanol)
.................................................... • solvent (Mineralised methylated spirit is ethanol mixed with methanol and other toxic additives plus a purple warning dye. This is to make it undrinkable so it can be sold without excise duty.)
.................................................... • fuels (As fuels, alcohols are rather expensive. They are used in some picnic stoves, and in Brazil (where a cheap source of carbohydrate is available for fermentation) there has been a conversion programme to use ethanol more widely as a fuel, including the conversion of car petrol engines to run on ethanol.)
.................................................... • starting material in many industrial reactions including: manufacture of alkylhydrogensulphates used as detergents (section 22.1.1. and FIG. 22.1. plus section 26.6.1.), manufacture of carbonyls (section 22.1.12.i.).
Ethane-1,2-diol (ethylene glycol) is manufactured from ethene via epoxyethane. It is used as car antifreeze and in the manufacture of Terylene (section 26.6.2). In the mid-1980's it was also used irresponsibly to give "body" to certain European wines!:
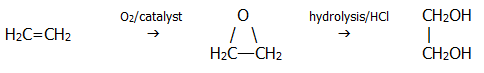
Phenol is used as a disinfectant and as the starting material in the manufacture of a large range of products including Nylon, aspirin, and phenolic resin plastics.
Cellulose forms rayon and guncotton by virtue of its hydroxyl groups (section 25.3.6.)
Ethoxyethane has been used as an anaesthetic and as a solvent.
26.5. CARBONYLS
26.5.1. Manufacture: Methanal is by made dehydrogenation of methanol (section 22.1.12.i.). Ethanal and propanone are also made by dehydrogenation though the main source of propanone is from the manufacture of phenol where it is a by-product, and most ethanal is obtained by catalytic oxidation of ethene. The manufacture of benzenecarbaldehyde has been covered in section 21.6.8.ii.
26.5.2. Two uses have already been given (section 22.2.6.vi.a.). Polymers of methanal (formed by evaporation of an aqueous solution) are high-strength plastics actually used to replace metal parts such as gear wheels and clips.
Propanone is used as a solvent, as is butanone which is found in paints and adhesives.
26.6. CARBOXYLIC ACIDS
AND THEIR DERIVATIVES
26.6.1. Soaps are important derivatives of long chain carboxylic acids, which, in turn, come from naturally occurring fats and oils, themselves esters (section 25.3.2.).
Detergents may also have the same ultimate source. The long chain carboxylic acids from fats and oils may be hydrogenated catalytically to long chain alcohols. These in turn may be converted to the alkylhydrogensulphate esters (sections 26.4.2. and 22.1.1. plus FIG. 22.1.). It is the sodium salts of these compounds which are used as detergents.
Another type of detergent is derived from alkanes (sections 26.2.5. and 21.2.1. plus Table 21.1.). Again it is the salts which are used as detergents.
26.6.2. The
action of soaps and detergents depends on their ionic,
water-soluble heads, and their long-chain, oil-soluble tails. This
allows them to disperse grease and oils in water as, so-called,
micelles.
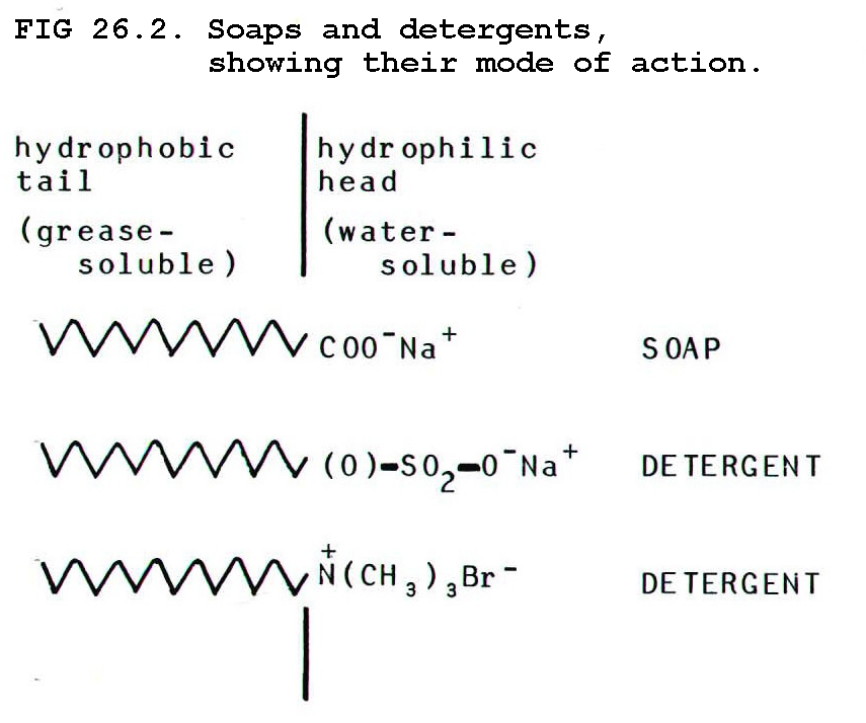
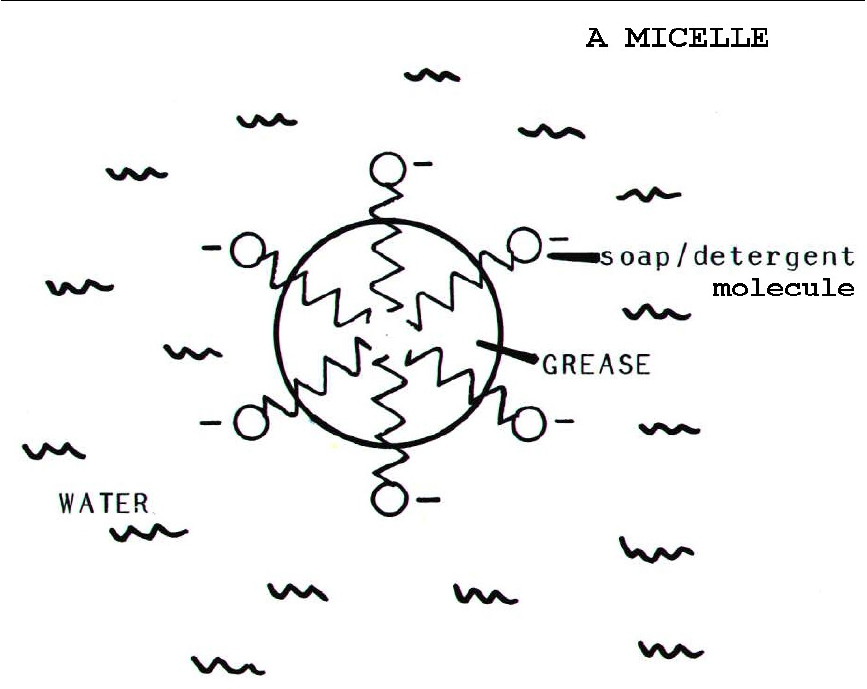
Soaps form insoluble calcium and magnesium salts which therefore
precipitate from hard water as scum. Detergents do
not form scum because their calcium and magnesium salts are soluble.
They are therefore more useful than soaps when used with hard water.
26.6.3. Condensation polymers: There are two main types of condensation polymer made from carboxylic acid derivatives: polyesters and polyamides.
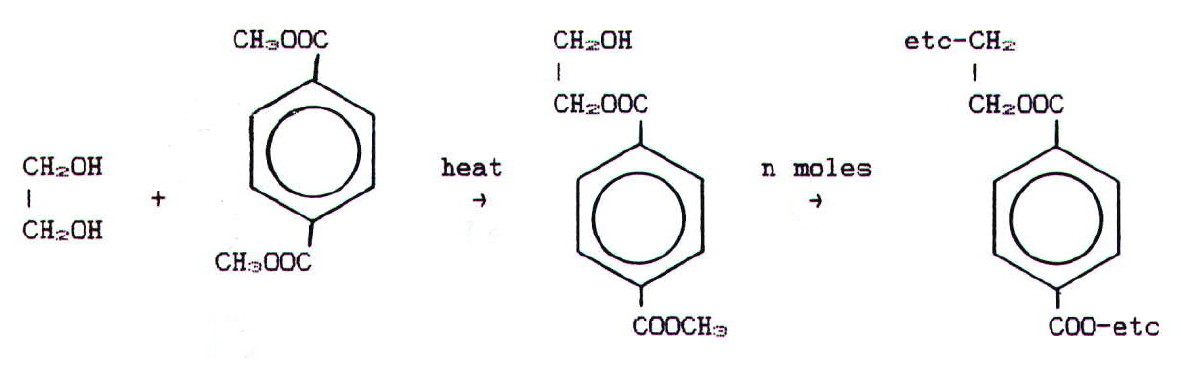
i) Polyesters: A
well-known polyester, Terylene, is made by heating ethane-1,2-diol with
dimethylbenzene-1,4-dicarboxylate (dimethyl terepthalate):
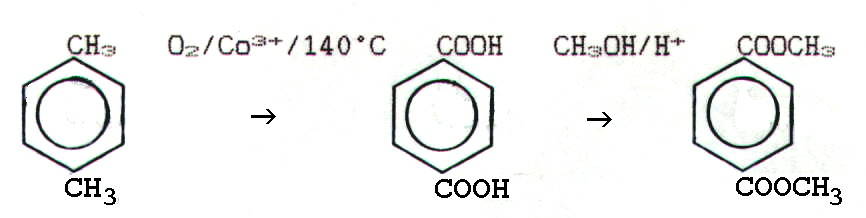
The dimethlybenzene-1,4-dicarboxylate is obtained via the following
reaction pathway:
The manufacture of ethane-1,2-diol has already been described in
section 26.4.2.
ii) Polyamides include Nylon 6, and Nylon 66. Nylon 6 is a polymer of one 6-carbon amino acid. Nylon 66 is a polymer of a 6-carbon diol and a 6-carbon diamine. Hence the names.
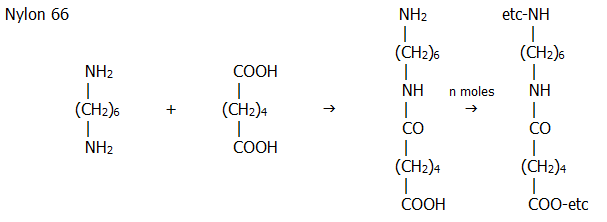
Both the
hexane-1,6-dioic acid and the diamine are obtained from phenol via the
following reaction pathways:
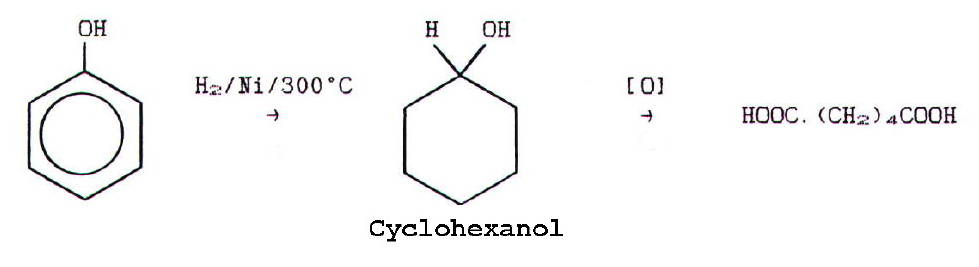
The hexane-1,6-diamine is then obtained by further reaction of some of
the diacid:
.
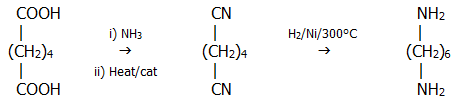
Nylon 6 is effectively
a polymer of 6-aminohexanoic acid. However, it is made from phenol via
the following reaction pathway:
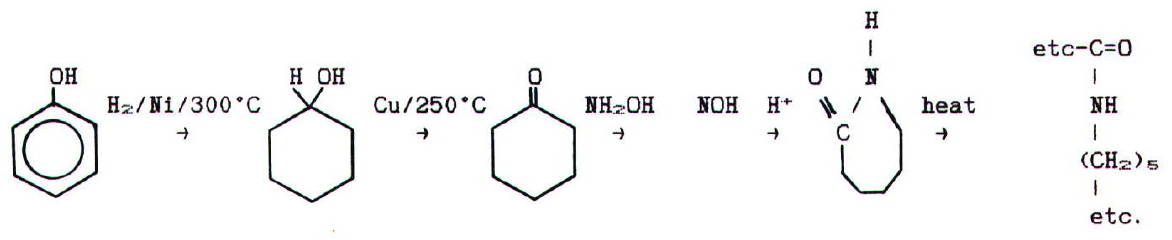
It may strike you that nylon 6 is very like a protein. In fact,
naturally occurring proteins have been used as plastics. Some of those
hard plastic buttons found on old clothes are made of casein, the
protein from milk.
26.6.4. Some further everyday occurrences and applications of carboxylic acids and their derivatives include:
• the occurrence of methanoic (formic) acid in the bite of ants and the sting of stinging nettles
• the occurrence of ethanedioic (oxalic) acid in rhubarb leaves, making them poisonous
• the use of methanoic acid as a preservative (e.g. grain preservative) and in textile processing
• the bacterial oxidation of ethanol to ethanoic acid i.e. vinegar
• the use of benzoic acid as a food preservative
• the industrial manufacture of ethanoyl anhydride from ethanal or from ethanoic acid (though not directly by elimination of water) and its industrial use as an ethanoylating agent e.g. in the manufacture of aspirin from 2-hydroxybenzoic acid or in the formation of "Tricel" from cellulose (3 ethanoyl groups per glucose residue).
• the flavour and smell of many fruits and flowers are caused by mixtures of esters. Also, mixtures of *synthetic esters may be used as artificial flavourings. *Or increasingly, mixtures of naturally occurring esters are used to flavour foods (section 28.3.1.), owing to the not very well thought out belief that naturally occurring flavours are automatically less harmful than artificial flavours.
• ethyl ethanoate is used as a solvent in some glues and lacquers
26.7.
NITROGEN-CONTAINING COMPOUNDS
The occurrence and industrial importance of nitrogen-containing compounds has been discussed in previous sections:
22.5.2. Manufacture of dyes
(25.2.) and 26.6.3.ii. Proteins as plastics
25.3.6. Manufacture of guncotton. A far more widely used explosive than gun cotton is 1-methyl-2,4,6-trinitrobenzene (Trinitrotoluene, or TNT).
26.6.3.ii. Manufacture of polyamide plastics.
26.8. CROSS-LINKING IN
POLYMERS
The bonds between one polymer and another affect its properties and uses. Polymers which are used as fibres must have fairly strong bonds between chains, such as the hydrogen bonding between parallel polyamide fibres and between parallel polyester fibres. Van der Waals bonding also exists, but is less important than the stronger hydrogen bonds.
In contrast, van der Waals bonding is the only type of bonding between polythene molcules and it is consequently not useful as a synthetic fibre.
26.9. QUESTIONS
1) Use one example of each to explain what is meant by the terms addition polymer and condensation polymer. Choose examples where you can supplement your answer with mention of everyday uses with which you are familiar.
2) Rayon (23.5.6.) and Tricel (26.6.4.) have repeating ester groups along the length of their molecules and are therfore examples of polyester plastics. Comment.
3) Ethanoic anhydride is used in preference to ethanoyl chloride as an industrial ethanoylating agent. Why do you think this is the case? Give at least two completely different reasons.
4) The organic chemical industry is not soley concerned with the manufacture of plastics. Discuss.
Unless otherwise stated, all materials in this web version of chapter
26 are © 2007 Adrian Faiers MA (Oxon) MCIPR

What 's the connection between a dozen eggs and
a garden mole?

Answer: Not a lot, really, but see Chapter 1