Recommended by:
Top
20 UK science resources,
The Tutor Website
Recommended by:

Rated:

2010
Listed on the science,
engineering
and technology section of 
'providing you with access
to the very best Web resources for education and research, evaluated
and selected by a network of subject specialists.'
(Please note that intute closed in July 2011)
Section 3: Organic
CHAPTER 27:
NOMENCLATURE PLUS BRIEF STRUCTURES AND
PHYSICAL PROPERTIES OF SOME ORGANIC COMPOUNDS
NB This chapter has now been
updated to improve browser compatibility.
Please
use the 'send email' link at the top right hand corner of this page to
report any problems.
27.1. ALKANES
Straight chain alkanes are named according to the number of carbon atoms:
| chain length | name | chain length | name |
| CH4 | methane | 5 carbon | pentane |
| CH3CH3 | ethane | 6 carbon | hexane |
| CH3CH2CH3 | propane | 7 carbon | heptane |
| CH3CH2CH2CH3 | butane | 8 carbon: | octane etc. |
Akyl groups are named methyl, ethyl etc.
In branched chain alkanes, the longest unbranched chain is found first, and named accordingly. The position of alkyl groups attached to the main chain is indicated by the number of the carbon atom (in the longest chain) to which they are attached. The name of the side group is given after this number and before the name of the main chain:
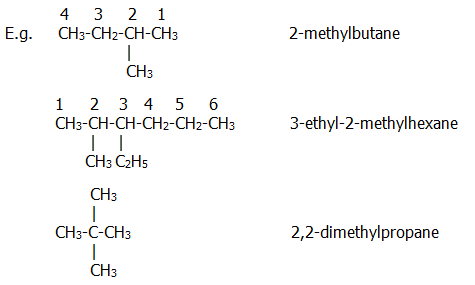
2,2-dimethylpropane can also simply be called dimethylpropane, because two methyl groups attached to carbon atom 2 is the only possible structure of a propane chain with two methyl groups attached to it. Think about what it would be called if one of the methyl groups were attached to a different carbon, given the longest carbon chain would then be a four carbon chain.
Note that: i) Names are all joined together in one long word.
ii) Hyphens are placed between numbers and words, and commas between numbers.
iii) Numbering of the carbon atoms in the longest chain is started at the end closest to a substituent/side chain.
iv) When there is more than one substituent/side chain they are named in alphabetical order.
v) When there are substituents/side-chains at equal distances from either end of the longest chain, the numbering is started at the end nearest to the first-named (alphabetical) substituent.
Branched alkyl groups are named accordingly:
......... CH3
.......... \
........... CH-..........................................
prop-2-yl
.......... /
......... CH3
Physical properties: C1 to C4 are colourless gases, C5 to C16 are colourless liquids, and higher alkanes are solid. Branching reduces melting point and boiling point by reducing the closeness with which the molecules can pack and thus reducing the extent of van der Waals bonding. Alkanes are generally soluble in organic solvents, but not in water.
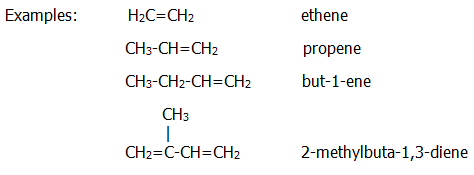
27.2. ALKENES
The carbon
chain is named in the same way as it is in alkanes, but
"-ane" is replaced by "-ene" to indicate the presence of a double bond.
The position of the double bond in a chain with more than three carbon
atoms is indicated by numbering:
Physical properties: The first four alkenes are colourless gases. Alkenes are insoluble in water but soluble in organic solvents.
27.3. ALKYNES
The naming of alkynes is essentially the same as the naming of alkenes except that the suffix "-ene" is replaced by "-yne".
...................
HC CH.............................
ethyne
CH.............................
ethyne
...................
CH3C CH.........................
propyne
CH.........................
propyne
Physical properties: Both the above alkynes are colourless gases which are insoluble in water but soluble in organic solvents.
27.4. BENZENE AND ITS DERIVATIVES
The structure of benzene is disccussed in chapter 3.
When there is more than
one substituent in a benzene ring, their relative positions are
indicated by numbering. The numbering is chosen to give substituents
the lowest possible numbers and, when relevant, they are placed in
alphabetical order:
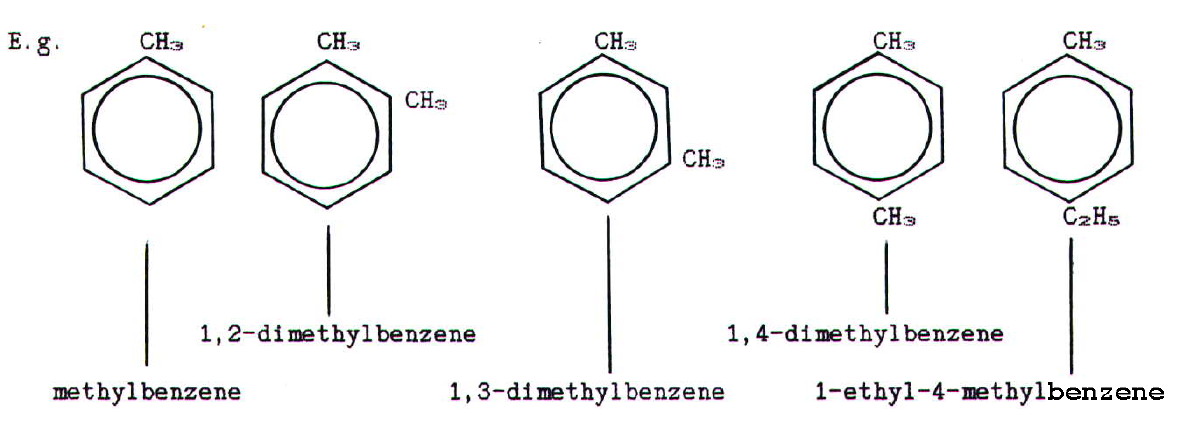
Note that carbon-2 used to be known as the ortho- (o-)
position, relative to carbon-1 in a disubstituted compound. Similarly
carbon-3 was known as the meta (m-) position, and
carbon-4 as the para (p-) position.
Sometimes, it is less
clumsy to name a benzene derivative "the other way round" in which case
the benzene group is called a phenyl group.

Physical properties: Benzene and the
derivatives shown here are all volatile liquids which are insoluble in
water and miscible with organic solvents.
27.5. HALOGEN COMPOUNDS
Halogen compounds are named systematically as halogen substituted hydrocarbons:
E.g................ CH3CH2Cl............. chloroethane, a primary haloalkane
.................... CH3
.....................
\
......................
CH-Br..................
2-bromopropane, a secondary haloalkane
.....................
/
....................
CH3
.................... CH3
....................
|
.............
CH3-C-I..................
2-iodo-2-methylpropane, a tertiary haloalkane
....................
|
....................
CH3
.................... CH2=CH-CH2Cl..... 1-chloroprop-2-ene
Trivial names such as ethylchloride are still
used. The terms primary, secondary and tertiary refer to the number of
alkyl groups attached to the functional carbon, except in halomethanes,
which are primary haloalkanes with only one carbon atom/alkyl
group.
Compare this with primary, secondary and tertiary alcohols (section
27.6.) and amines (section 27.10.). As a group, haloalkanes are also referred to as
halogenoalkanes. The trivial name for the group is alkyl
halides.
Physical properties: Chloromethane, bromomethane, and chloroethane are colourless gases at room temperature. Other halohydrocarbons are colourless liquids.
27.6. HYDROXY COMPOUNDS
Alcohols are named by using a suffix, "-ol", rather than a prefix like that used in the case of haloalkanes. (Systematic nomenclature is not as consistent as it might be.) The suffix replaces the "-e" in the parent alkane's name.
.......... CH3CH2OH...................... ethanol, a primary alcohol
..........
CH3
........... \
............
CHOH............................
propan-2-ol, a secondary alcohol
........... /
.......... CH3
.......... CH3
.......... |
.. CH3-C-OH.......................
2-methylpropan-2-ol, a tertiary alcohol
.......... |
.......... CH3
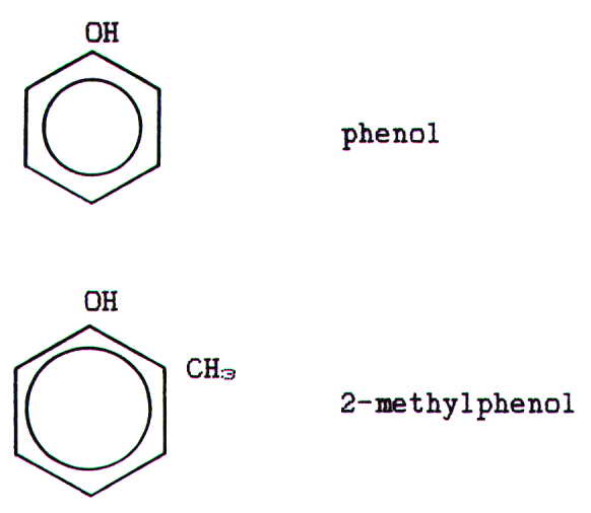
Physical properties: The lower alcohols are
colourless liquids with higher boiling points than might be expected.
This is a result of hydrogen bonding between molecules (section
22.1.7.ii.).
As well as being miscible with organic solvents, and provided the hydrophobic carbon chain is not too long, alcohols are miscible with water due to hydrogen bonding with water (section 22.1.7.). Phenol is also quite soluble.
2-methylpropan-2-ol and phenol are low melting point solids.
27.7. ETHERS
Part of the ether (-OR) is considered as an alkoxy group attached to the other (longer if there is a difference in chain length) R group:
E.g............ CH3
..................
\
...................
O...........................
methoxyethane
..................
/
........... CH3CH2
The most common ether, ethoxyethane, is also still known as diethylether, or simply as ether.
Physical properties: Ethers are liquids, but far more volatile than the alcohols with which they are isomeric. This is because there is no possibilty of hydrogen bonding. They are also immiscible with water.
27.8. CARBONYLS
i) Aldeyhdes
are named using the usual prefixes to indicate cahin length, and the
suffix "-al" to indicate the aldehyde group. Note that aldehyde groups
can only be terminal:
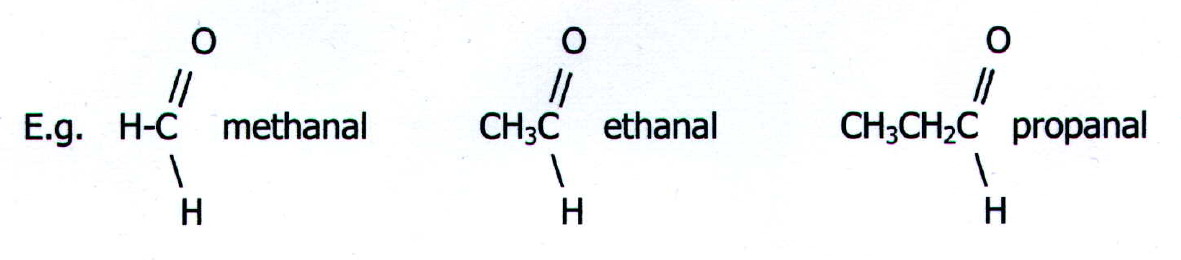
Methanal is still regularly called formaldehyde, and ethanal is often
called acetaldehyde.
Aromatic aldehydes are named by adding the suffix carbaldehyde to the name of the parent hydrocarbon. Thus C6H5CHO should strictly be known as benzenecarbaldehyde, but is usually called benzaldehyde.
ii) Ketones are again named using the usual prefixes. The ketone group is denoted by the suffix "-one", and the position of the group is indicated by numbering of the carbon chain.
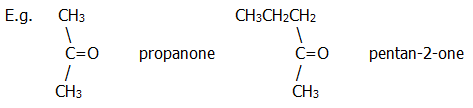
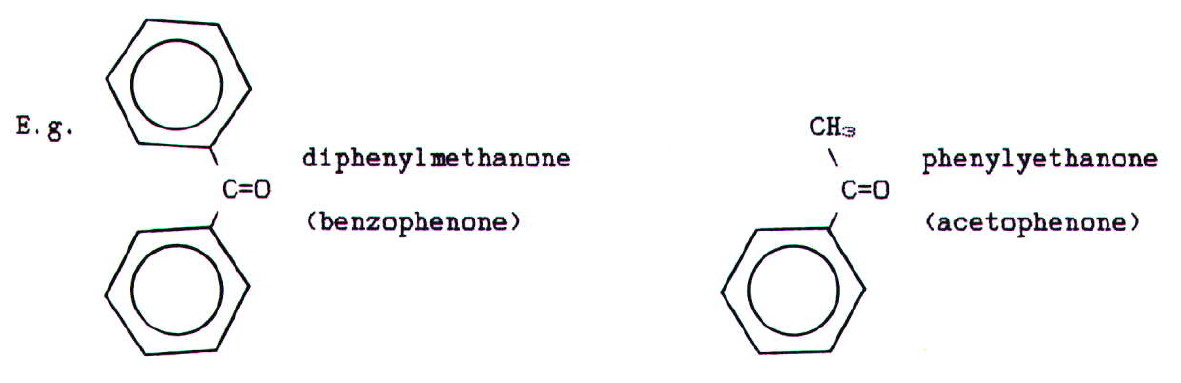
Aromatic ketones tend,
even now, to be known by their trivial rather than systematic names:
Physical properties: Methanal is a colourless
gas, but the other carbonyls are colourless liquids. Methanal has a
sharp but musty smell. Ethanal has a sharp smell described as being
like fresh apples or tomatoes. The lower carbonyls are soluble in /
miscible with water, as well as organic solvents. A 40% solution of
methanal in water is the preserving solution "formalin".
27.9. CARBOXYLIC ACIDS AND THEIR DERIVATIVES
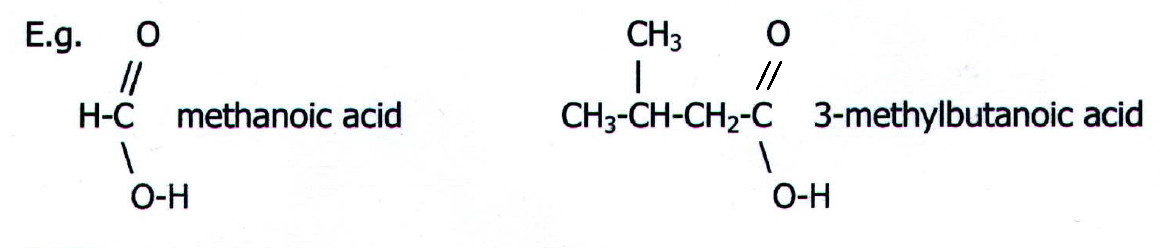
27.9.1. Carboxylic
acids: The aliphatic carboxylic acids are named after the
corresponding alkanes, the suffix "-oic acid" replacing the "-e".
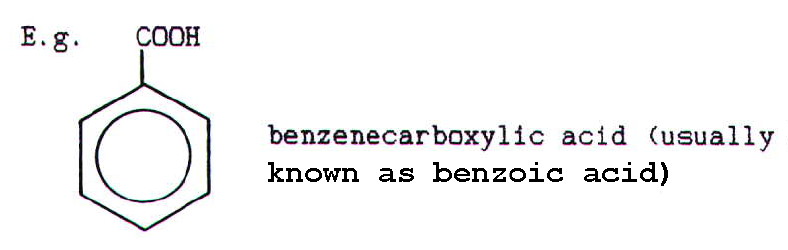
Aromatic acids are named using the name of the hydrocarbon followed by
"carboxylic acid" to indicate the presence of the acid group:
Physical properties: The lower carboxylic
acids are colourless liquids which are miscible with water. The
solubility in water is due to hydrogen bonding between the carboxylate
group and water. This is increasingly counteracted as the length of the
hydrophobic carbon chain increases. Their boiling points are higher
than expected due to association between pairs of molecules (section
22.3.4.)
27.9.2. Carboxylic
acid derivatives are named reasonably logically, as can be
seen from the following examples. The examples also show the structures
of the functional groups:

the smallest acid chloride. It is a colurless liquid which reacts with
water (22.3.6.i)
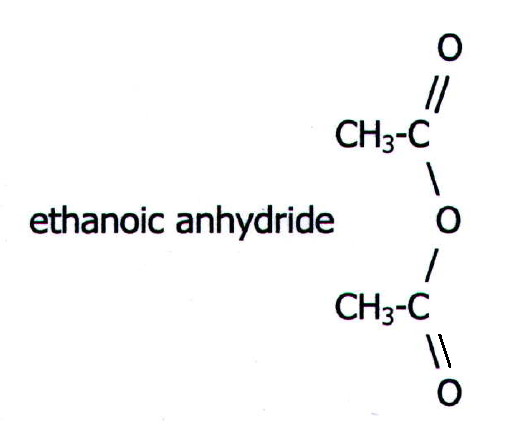
the smallest acid anhydride. It is a colourless liquid which reacts
with water (22.3.6.i.).
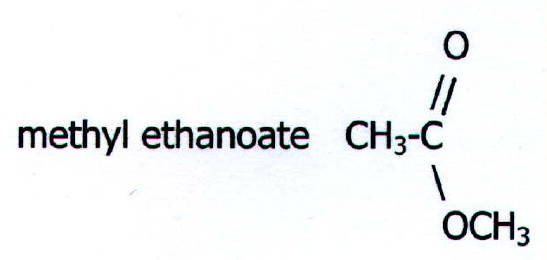
an ester. If you find it difficult to decide which part of the name
refers to which alkyl chain, compare with sodium ethanoate. The simpler
esters are colourless volatile liquids with pleasant, often fruity,
smells. They are insoluble in water but react with it on prolonged
heating (22.3.6.i.)
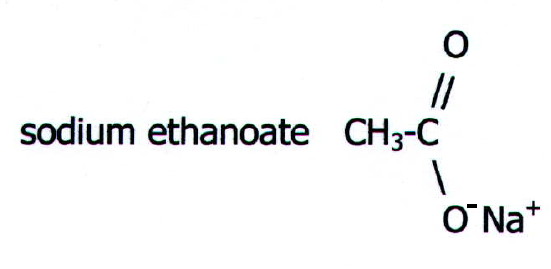
a salt.
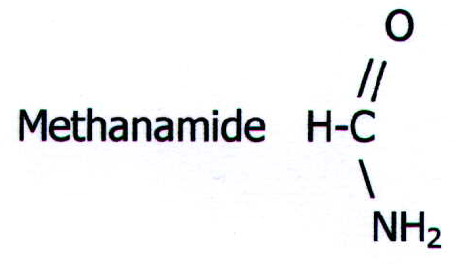
the smallest amide. Methanamide is a high boiling point colourless
liquid, the others are white solids. The lower members are soluble in
water.
For names of aromatic derivatives see study question 7.
27.10. AMINES
Amines divide into primary, secondary, and tertiary amines, but the terms describe the number of alkyl groups attached to the functional nitrogen. Compare this with the terms as applied to haloalkanes and alcohols (sections 27.5. and 27.6.). With amines, the series is completed by quarternary ammonium salts. All the members of the series are named according to the alkyl groups present:
E.g........... CH3NH2............ methylamine, a primary amine
.................CH3
..................\
...................NH..................
dimethylamine, a secondary amine
........... ....../
................CH3
................CH3
.................\
...........CH3-N......................
trimethylamine,
a tertiary amine
................./
...............CH3
..............CH3
....+
...............|
.......H3C-N-CH3....... Cl-..... tetramethylammonium
chloride,
...............|.........................a
quarternary ammonium salt.
..............CH3
...............C6H5NH2............ phenylamine, a primary amine
...............C6H5CH2NH2...... (phenylmethyl)amine, a primary amine
...............C6H5NHCH3........ N-methylphenylamine, a
secondary amine
BUT:
...............CH3
................\
................CH-NH2............. 2-aminopropane, a
primary amine
.............../
..............CH3
Physical
properties: Methylamine, ethylamine, and dimethylamine are
colourless gases, the other lower amines are colourless liquids.
Association via hydrogen bonding gives them higher boiling points than
might be expected:
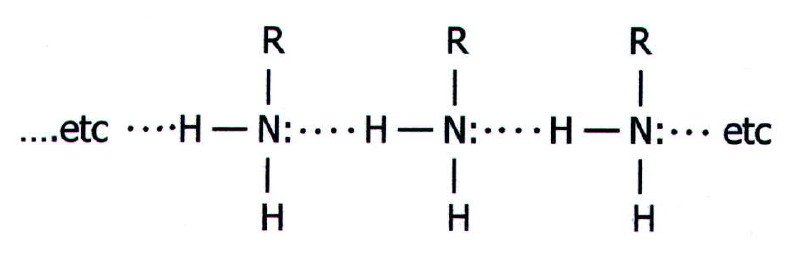
They tend to have a fishy smell, and the lower amines are soluble in
water, again due to hydrogen bonding.
27.11. NITRILES
The suffix "-nitrile" is added to the whole of the name of the relevant alkane:
E.g........ CH3-C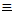 N.......
ethanenitrile
(methyl cyanide)
N.......
ethanenitrile
(methyl cyanide)
..............CH3-N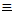 C.......
methaneisonitrile
(methylisocyanide)
C.......
methaneisonitrile
(methylisocyanide)
Aromatic nitriles are named after the parent hydrocarbon plus "carbonitrile"):
E.g........ C6H5C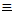 N.......
benzenecarbonitrile
(benzonitrile)
N.......
benzenecarbonitrile
(benzonitrile)
Physical properties: Nitriles are liquids which are largely immisible with water, but slowly hydrolysed by it. Isonitriles have a foul smell.
27.12. NITRO COMPOUNDS
Are named in a similar
way to halocompounds:
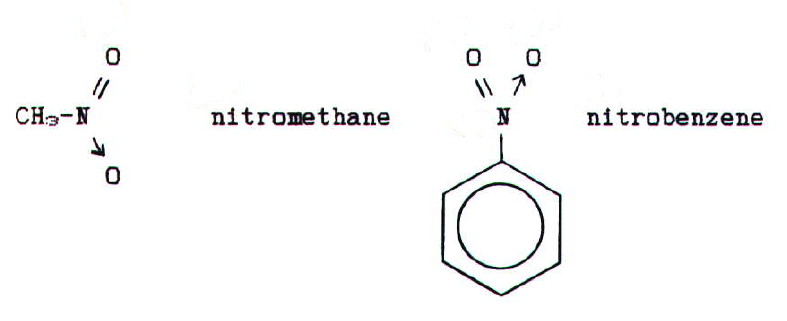
Physical properties: Nitro compounds are
liquids which are largely immiscible with water, but slowly hydrolysed
by it. Nitrobenzene smells of almonds.
Note that nitroalkanes are isomeric with alkyl nitrates (R-O-N=O) which are inorganic esters of nitric(III) acid.
27.13. QUESTIONS
1) Look at the structures of all the main classes of compound listed in this chapter. Describe clearly in words the characteristic part of the structure for each class of compound.
2) Using the information in this chapter, together with information in
chapter 3, draw orbital overlap structures for: methanal, benzenecarbaldehyde, methanoic acid, nitrobenzene.
Note that, when applicable, your diagrams will give you a fuller understanding of delocalisation.
3) Systematic nomenclature is not as consistent as the name implies. Comment.
4) Account for the fact that phenol is less soluble than ethanol in water, yet phenol more readily dissociates into ions?
5) From the examples given in section 27.9.2., describe the simple rules governing the naming of carboxylic acid derivatives. The sentence describing the naming of carboxylic acids (section 27.9.1.) gives the format.
6) Predict the physical properties of sodium ethanoate.
7) Write structures for phenylbenzenecarboxylate, benzenecarboxamide, phenylethanoylchloride, 2-phenylpropanamide, and N-phenylpropanamide.
8) Write structures for propan-2-ol, 2-aminobutane, 2-chloro-2-methylpropane, and 2-amino-2-methylpropane, stating whether the compounds are primary, secondary, or tertiary.
Unless otherwise stated, all materials in this web version of chapter
27 are © 2007 Adrian Faiers MA (Oxon) MCIPR

What 's the connection between a dozen eggs and
a garden mole?

Answer: Not a lot, really, but see Chapter 1