Recommended by:

Top 20 UK science resources,
The Tutor Website
Recommended by:

Rated:

2010
Listed on the science, engineering and technology section of

'providing you with access to the very best Web resources for education and research, evaluated and selected by a network of subject specialists.'
(Please note that intute closed in July 2011)
Section 1: Essentials (physical chemistry)
CHAPTER 2: ATOMIC
STRUCTURE (more detail)
NB This chapter has now been
updated to avoid the need for non standard fonts such as Wingdings 3.
Please use the 'send email' link at the top right hand corner of this
page to report any problems.
QUICK SKIPS:
(Click on the 'back' arrow to get back to this quick skip section)
Models
Atomic spectra
Orbitals
The electrostatic
approach
Predicting electron
arrangement
Electron
arrangement and the periodic table
More information from
the
periodic table
Predicting
properties
Revision
worksheet 1: Atomic
Structure
Answer sheet 1:
Atomic Structure (follow link on home page)
2.1. INTRODUCTION
2.1.1. Once the number of neutrons, protons, and electrons in an atom has been deduced, it is possible to construct models which represent the arrangement of these fundamental particles.
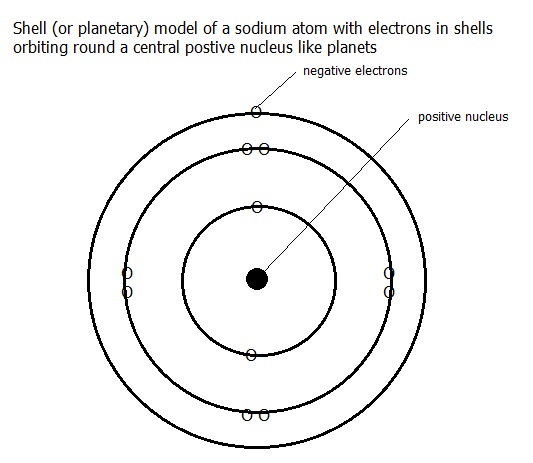
At elementary levels of chemistry a simple model of the atom is used in which the neutrons and protons are placed in a nucleus at the centre of the atom. The electrons are considered as occurring in spherical shells around the nucleus. They are held in position by the electrostatic attraction between the positively charged nucleus and the negatively charged electrons.
The relative sizes of the nucleus and the electron shells are well illustrated by imagining a hydrogen nucleus the size of a pea on the centre spot of a large football stadium. The electron shell would only just be contained within the stadium.
2.1.2. Usefulness of the model: When considering much of an atom's behaviour, this model is still useful at this level. It should not be considered as incorrect any more than FIG. 2.1. should be considered an incorrect model of an aircraft wing.
This model of a wing is useful when illustrating simple aerodynamic principles. It does not look as much like an aircraft wing as the relevant part of a polystyrene construction kit, but the latter model serves a different purpose, and may be too complex for simple aerodynamics.
Similarly, at higher levels of chemistry we encounter models of the atom which probably look more like the real thing (section 2.3.). However, the shell model may be more helpful when trying, for example, to predict the effect of atomic size on the ease with which outer electrons are lost. Moreover, when writing equations, aphabetical letters are the most useful models of atoms.
It is also important to understand the balance between the extent to which models help us "explain" observations, and the extent to which they merely help us "describe, collate, and rationalise" them. An important role of theory is to unify (sections 4.4.5., 6.1.1. and 10.2.1.).
Also, by helping us describe, collate, and rationalise observations, models help us to make predictions. In other words, using the clarified understanding we gain from a model, we may be able to describe behaviour in advance.
We only "explain" behaviour when, for example, the model describes the behaviour in terms of:
...........................i)
more
fundamental properties,
.....................or
.ii) more
detailed
properties,
.....................or
iii)
more general properties.
In such cases we can cautiously use words like "because" and talk about cause and effect. Even then, the description of the behaviour may be greater than actual explanation.
For example, we may have a model of electrostatic attraction which has positive charge attracting negative charge. At one level we can explain the attraction of protons for electrons by saying that they are oppositely charged. At another level, we are merely describing the behaviour by saying that the attraction is consistent with previous observations on particles which we have classified as oppositely charged.
The key question is this: Does the concept of charge describe a property which is, for example, more fundamental, detailed, or general than the observed property of attraction (study question 1)? Questions like this are absolutely essential in order to keep theory relevant to observation and experiment, and in order to keep scientific method more generally in perspective. Moreover, just because a prediction made by applying a model turns out to be confirmed experimentally does not mean that the model is 100% correct; it does mean that it works - it is useful.
Few scientists are philosophers. Even fewer are theologians! In these two respects, science has probably suffered as it has become more established.
2.2.1. The shell model is a response to information from atomic spectroscopy. This model has the protons and neutrons in a small nucleus at the centre of the atom with the electrons orbiting around the nucleus in shells. To give some idea of the word small in this context: if the nucleus were the size of a pea placed on the centre spot of a football pitch in a large stadium, the outer shell would be at the outside of the stadium.
If an electric current is passed through an element in gaseous form at low pressure, electromagnetic radiation is emitted. When the radiation is analysed in more detail it is seen to be emitted only at certain precise frequencies. The radiation is observed as a, so-called, emission spectrum.
Similarly, atoms of an element can absorb electromagnetic radiation, but only at the same precise frequencies as those observed in emission spectra. This produces an absorption spectrum.
In response to these data, it has been proposed that the radiation has precise energy values (related to frequency: v=fl, E=hf) corresponding to movements of electrons between precise energy levels around the nucleus (pictured as spherical shells in the shell model).
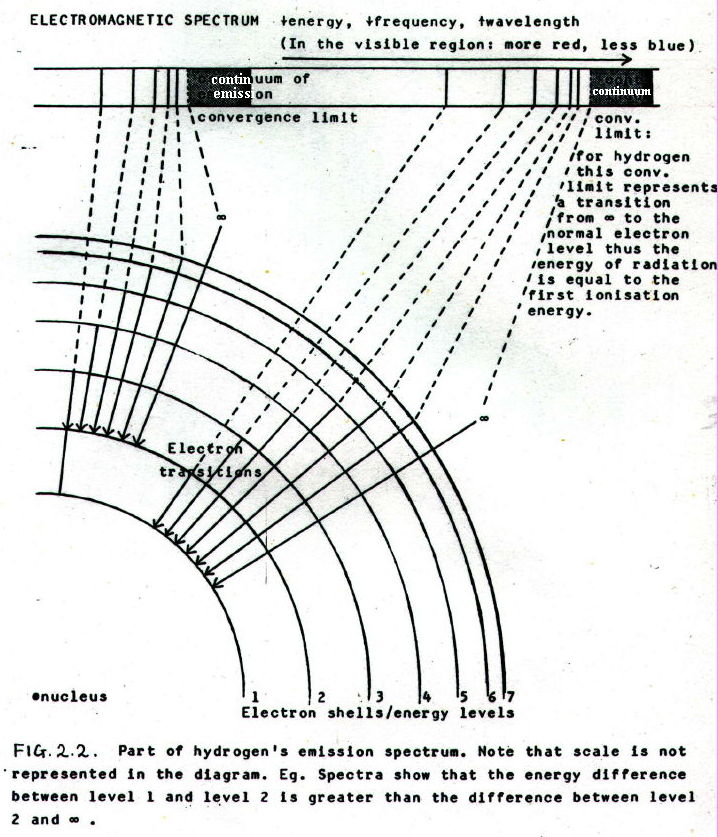
It is proposed that emission spectra occur when electrons are excited into higher energy levels (e.g. by an electric current) and on returning from higher to lower levels, they emit electromagnetic radiation. The energy of the radiation emitted is equal to the energy difference between levels.
Conversely, when electromagnetic radiation is passed through the gas, it is suggested that electrons are excited to higher energy levels by absorbing radiation with exactly the energy difference between the two levels. This produces an absorption spectrum because on returning to lower levels, the electrons release energy as heat.
Both these processes produce line spectra. For example, in the visible region, light produced by an emission spectrum and split by a prism would be observed as bright lines of particular colours. In the case of an absorption spectrum, dark lines of "missing light" would be seen.
By contrast, when light is produced by heating a solid or liquid, the spectrum is continuous like sunlight. When sunlight is split by a prism, the characteristic rainbow series of colours is produced.
2.3.1. More finely resolved atomic spectra show that the proposed energy levels are more finely divided than described by the shell model. A model consistent with this extra information has the electron shells (energy levels) divided into sub-levels known as orbitals. Each orbital can hold a maximum of two electrons.
In pictorial terms, an orbital is the geometric space around the nucleus in which up to two electrons with a particular energy and opposite spins are most likely to be found. Bearing in mind the dual particle/wave nature of electrons, orbitals are best imagined as regions of electron density.
2.3.2. There are four types of orbital, s, p, d, and f. Each has a characteristic shape shown in FIG. 2.3.
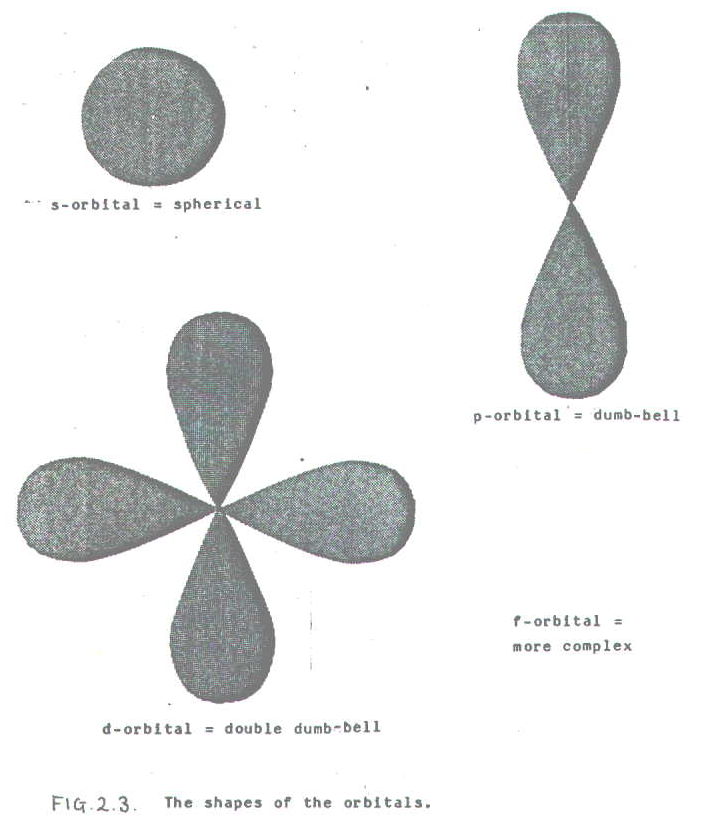
At A-level you are required to know the shapes of only s and p orbitals. Moreover, one of the d-orbitals is more like a single dumbell with a doughnut round the middle.
There is one s-orbital per energy level. Then, from the second level onwards, there are three p-orbitals per energy level. From the third level onward, there are five d-orbitals per energy level. Finally, from the fourth level onwards, there are seven f-orbitals per energy level. Given the maximum number of electrons per orbital it is therefore also possible to work out the number of electrons per s, p and d sub-shell as two, six and ten respectively. (See also the final paragraph in section 2.4.1 below.)
In a given energy level, the orbitals of a particular type are initially equivalent in energy. However, the types decrease in energy in the order f > d > p > s.
2.3.3. Electrostatic approach: It would be far too great a break with tradition to talk solely about electrostatic attraction of the nucleus for the electrons in orbitals, rather than talking about their energy. Moreover, energy is an essential concept if we are to develop our models of atoms.
However, it is important to remember that when we talk about an electron with a high energy, we are talking about an electron which is not attracted very strongly by the nucleus. In the shell model such an electron is far from the nucleus and easily removed (a low amount of energy is needed to remove it).
Conversely, an electron with a low energy is attracted strongly by the nucleus. In the shell model it is close to the nucleus and difficult to remove (a high amount of energy is needed to remove it). See also section 14.4.2.v.
2.3.4. In some cases it is more convenient to represent orbitals by boxes, rather than drawing out their shapes:
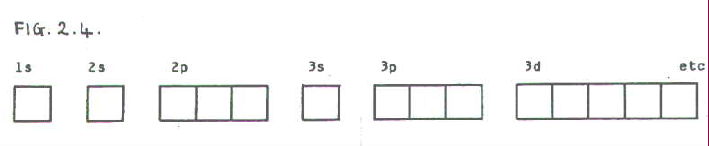
An even simpler notation shows the number of electrons in a particular set of orbitals by using an indice. E.g. If there are four electrons in the 2p orbitals, this is shown as 2p4.
In the box notation electrons are shown as arrows. The arrow head represents a spin direction, and if there are two electrons in one orbital, they are shown to have opposite spins. E.g. 2p4:
2p4...........
2.4.
PREDICTING THE ELECTRON ARRANGEMENT
2.4.1. Three rules: There are three important rules which describe the way in which electrons are distributed amongst the orbitals of a particular atom:
i) The electrons are found in the lowest energy orbitals available (Aufbau principle).
ii) Each orbital can hold a maximum of two electrons, and these have opposite spins (a version of Pauli's exclusion principle).
iii) Orbitals of equivalent energy (e.g. the three 2p orbitals) are occupied singly before pairing occurs (Hund's rule).
Clearly it is necessary to know the energy ranking of the different types of orbital in order to apply these rules. There is a gimmicky diagram which allows you to work out this ranking, but it is far more sensible to work it out from the layout of the periodic table. This is particularly true because the more familiar you are with the periodic table, the easier you will find large sections of physical and inorganic chemistry. Moreover, you are more than likely to be given a copy of the periodic table in exams!
In addition, understanding the periodic table and what it represents in terms of increasing number of protons, corresponding increasing number of electrons and arrangement in terms of sub-shells and orbitals, enables you to deduce, for example:
- the number of electrons that can fill each shell;
- the number of electrons filling s, p and d sub-shells
- the number of orbitals (containing a maximum of two electrons) per sub-shell.
2.4.2. Electron arrangement and the periodic table: The energy ranking of the orbitals is deduced by starting at the top left hand corner of the table and travelling across the periods, from left to right, one at a time (i.e. working through the elements in order of the number electrons they have, as worked out from atomic number which = number of protons).
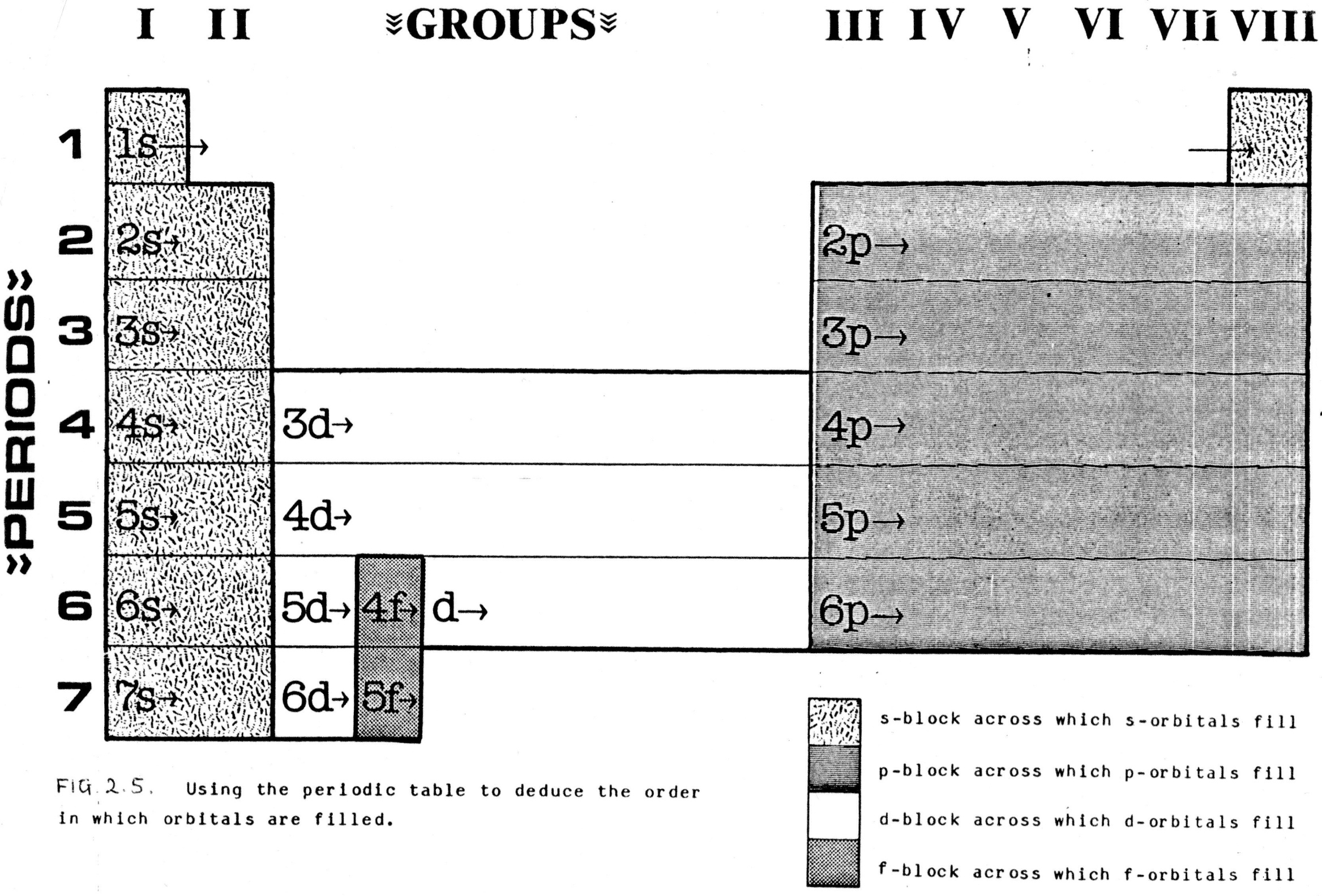
d-orbitals, which occur from the third energy level onwards, do not fill until the s-orbital in the next energy level is filled. This is why the energy level of the d-orbitals is always one behind the period number. For comparable reasons, the energy level of f-orbitals is always 2 behind the period number.
FIG.2.6. Electron configurations of some elements. Note the apparent idiosyncrasies at chromium and copper:
1H 1s1
2He 1s2
3Li 1s2 2s1
5B 1s2 2s2 2p1
15P 1s2 2s2 2p6 3s2 3p3
19K 1s2 2s2 2p6 3s2 3p6 4s1
21Sc 1s2 2s2 2p6 3s2 3p6 3d1 4s2
23V 1s2 2s2 2p6 3s2 3p6 3d3 4s2
24Cr 1s2 2s2 2p6 3s2 3p6 3d5 4s1
25Mn 1s2 2s2 2p6 3s2 3p6 3d5 4s2
28Ni 1s2 2s2 2p6 3s2 3p6 3d9 4s2
29Cu 1s2 2s2 2p6 3s2 3p6 3d10 4s1
30Zn 1s2 2s2 2p6 3s2 3p6 3d10 4s2
35Br 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5
The period number is equal to the number of the highest energy level (outer shell) of any atom in that period. It is somewhat confusing that orbitals in a generally higher energy level, may actually have a lower energy than those in the previous level.
A simple consideration of potassium, calcium, and scandium shows that the situation is a little more involved, even than this.
2.4.3. Interchanging energy levels: The highest energy electrons in potassium and calcium are found in the 4s orbital rather than in a 3d orbital. The 3d orbitals are empty, suggesting they have a higher energy than the 4s orbital.
In scandium, two of the three highest energy electrons are found in the 4s orbital and the third is found in the 3d orbital. So far this seems consistent. However, the 4s electrons have lower ionisation energies (section 6.6.2.iii.) than the 3d electron, suggesting that the 4s orbital is at a higher energy than the 3d orbital (section 2.3.3.).
Moreover, in titanium, the 4s electrons actually participate in bonding more readily than the 3d electrons.
All we are saying is that the 3d orbitals appear to have a higher energy than the 4s orbitals in potassium and calcium, but in scandium and titanium (and the other transition elements) the 4s orbitals appear to have a higher energy than the 3d orbitals.
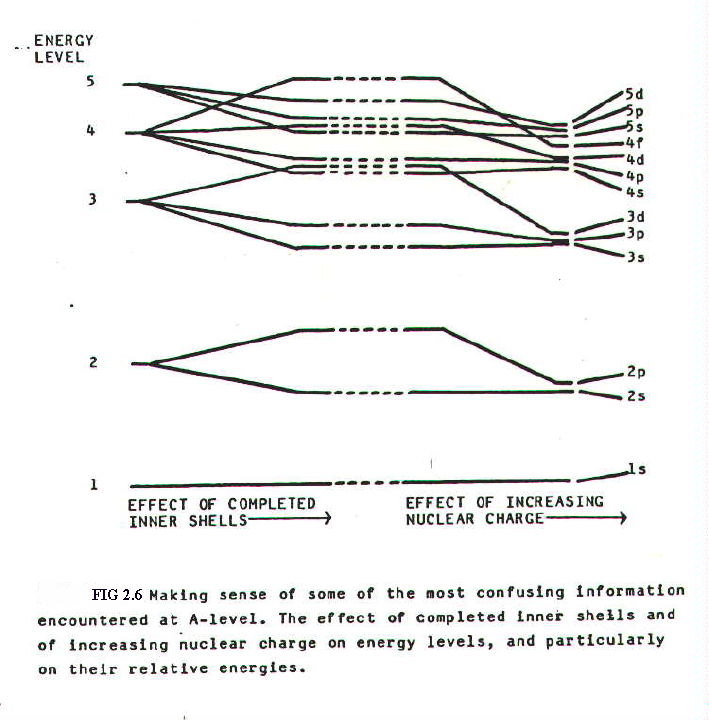
In fact this is the case. But we have come a long way without mentioning electrostatic attraction. The changeover in relative energies of the 3d and 4s orbitals is much easier to understand in terms of a model which emphasises electrostatic forces rather than energy: as soon as electrons are attracted into 3d orbitals, they repel 4s electrons away from the attraction of the nucleus.
Both nuclear charge and the filling of inner shells have a great effect on orbital energies as shown in FIG. 2.7.
2.5. MORE INFORMATION FROM THE PERIODIC TABLE
2.5.1. There is more information that can be deduced from atomic number, using an understanding of the periodic table. For example, take the element in period 5, group IV (atomic number 50):
i) Atomic number: Strictly speaking, position in the periodic table is deduced from atomic number, but in practice, it is the position in the periodic table which should be remembered. Atomic number can then be worked out from position in the table, by counting. It is therefore found to be 50 in this example.
ii) Number of shells: The period number immediately gives the number of energy levels, and the number of the highest energy level (outer shell). It is obviously 5 in this example
iii) Number of outer electrons: The group number immediately gives the number of outer electrons. It is obviously 4 in this example.
iv) Outer electron configuration: An understanding of the periodic layout (FIG. 2.5.) immediately indicates that the outer electron configuration in this example is:
5s2
5p2...........
iv) The rest of the electronic structure is also easily deduced from an understanding of the periodic table's layout:
.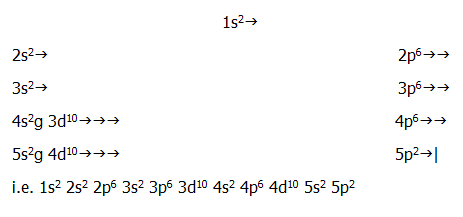
vi) Chemical properties: Since so many chemical properties depend on the number of outer electrons and the number of inner shells, even these properties may be deduced (chapters 13 to 18).
vii) Physical properties: These too depend on electron configuration. The relative atomic mass is also directly relevant to physical properties, but even this can be roughly estimated:
viii) Relative atomic mass: This can be roughly estimated once the atomic number has been deduced. The basis of the estimation is as follows.
Hydrogen has a sole proton in its only stable nucleus. From helium onwards, neutrons are needed in the nucleus to stabilise it, and after bismuth (atomic number 83) no number of neutrons will produce a stable nucleus.
The ratio of neutrons:protons needed to stabilise a nucleus increases from 1:1 for helium, to about 1.5:1 for 83Bi. A simple model is consistent with this fact. Protons are positively charged and therefore repel each other. Neutrons can be regarded as keeping them apart.
Thinking in only two dimensions, it can be seen that only two neutrons would be needed to keep two protons apart, whereas five would be needed to keep four protons apart (FIG. 2-7.jpg).
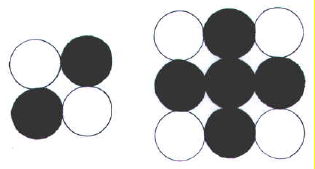
If we assume that the increase in ratio of N:P from 1:1 to 1.5:1 is linear, we can roughly estimate the number of neutrons in a nucleus from the atomic number.
E.g. for our example element which has an atomic number of 50:
2He....................................50X...................................83Bi
N:P.....................................N:P...................................N:P
1:1................1+(50/83 x 0.5):1.................................1.5:1
....................................= 1.3:1
....................................= 50 x 1.3 neutrons
....................................= 65 neutrons.
This gives 50 protons and 65 neutrons, giving an estimated RAM of 115. In fact this element is tin, which has a relative atomic mass of 118.7. The small difference is due to the existence of isotopes and due to the fact that the N:P ratio does not actually increase linearly (FIG. 3.1.)
The RAM of 118.7. further draws attention to the fact that atoms of an element may occur as more than one type (isotopes) with differing numbers of neutrons. Obviously, the number of protons is the same in all atoms of an element. This is predictable because it is the number of protons (and more directly the number of electrons, especially outer electrons) which determines the element's characteristic properties.
Even below an atomic number of 83, unstable isotopes of an element may exist if the ratio of neutrons to protons is not appropriate. This is the starting point of the next chapter.
2.6. QUESTIONS
1) Does the concept of charge describe a property which is more fundamental, detailed, or general than the attraction between a proton and an electron? For example, can you think of a case of attraction between opposite charges that does not ultimately depend on attraction between protons and electrons? Thus to what extent does charge difference explain the attraction between protons and electrons, and to what extent does it merely describe it?
2) Describe an orbital model of the atom which emphasises electrostatic attraction between nuclei and electrons rather than the energies of orbitals.
Unless otherwise stated, all materials in this web version of chapter 2 are © 2007 Adrian Faiers MA (Oxon) MCIPR

What
's the connection between a dozen eggs and a garden mole?

Answer: Not a lot, really,
but see Chapter
1