Recommended by:

Top 20 UK science resources,
The Tutor Website
Recommended by:

Rated:

2010
Listed on the science, engineering and technology section of

'providing you with access to the very best Web resources for education and research, evaluated and selected by a network of subject specialists.'
(Please note that intute closed in July 2011)
Section 1: Essentials (physical chemistry)
CHAPTER 3:
RADIOACTIVITY
NB This chapter has now been
updated to improve browser compatibility.
Please
use the 'send email' link at the top right hand corner of this page to
report any problems.
QUICK SKIPS:
(Click on the 'back' arrow to get back to this quick skip section)
Band of Stability
Predicting type of
radioactivity
Kinetics of radiation
Applications of
radioactivity
3.1. BAND OF STABILITY
3.1.1. All nuclei with more than 83 protons are unstable. Elements with 83 protons or fewer may also have unstable nuclei, depending on the ratio of neutrons to protons.
If number of neutrons is plotted against number of protons for stable nuclei, a characteristic graph is obtained (FIG. 3.1.).
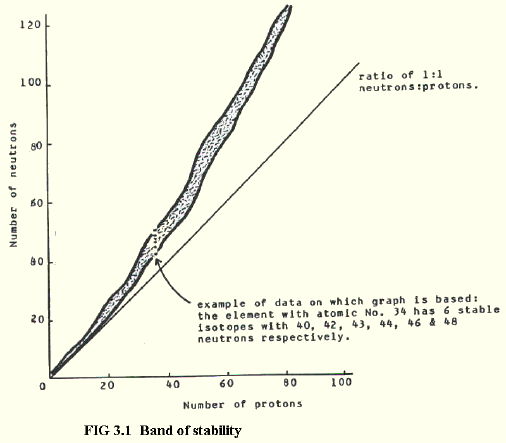
The plot produces a band of stable nuclei called the band of stability. A section of the band is shown in more detail at element 34, which has six stable nuclei.
Elements which lie
outside the band undergo radioactive decay. This
produces a new nucleus which may or may not be radioactive itself. The
process continues until a stable nucleus is formed. This can be seen
more clearly by understanding nature of three main types of radiation.
3.2. 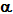 ,
, 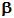 , AND
, AND 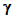 RADIATION
RADIATION
3.2.1. Nature
of 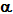 radiation: This is the loss by an unstable nucleus of
two protons and two neutrons as a single
radiation: This is the loss by an unstable nucleus of
two protons and two neutrons as a single 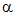 -particle. An
-particle. An 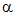 -particle is therefore a helium nucleus:
-particle is therefore a helium nucleus:
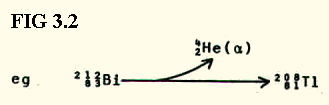
Note that the ratio of neutrons:protons changes because they are removed in a different ratio from that which exists in the parent atom.
However, the Thallium
nucleus produced by the decay is still unstable and it must undergo the
second type of radiation (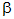 ) before a stable nucleus results.
) before a stable nucleus results.
3.2.2. Nature
of b
radiation: When 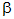 -radiation occurs, the ratio of
neutrons:protons is reduced because a neutron changes into a proton. At
the same time an electron is produced, and this is lost from the
nucleus as a, so-called,
-radiation occurs, the ratio of
neutrons:protons is reduced because a neutron changes into a proton. At
the same time an electron is produced, and this is lost from the
nucleus as a, so-called, 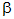 -particle:
-particle:

3.2.3. Nature
of 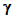 -radiation: During
-radiation: During 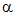 - and
- and 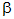 -decay,
excess energy may be released as high frequency electromagnetic
radiation known as
-decay,
excess energy may be released as high frequency electromagnetic
radiation known as 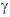 -radiation.
-radiation.
3.3. PREDICTING THE
TYPE OF RADIATION
3.3.1. Up to
element 82, nuclei which have too high a ratio of
neutrons:protons undergo 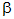 -radiation, but not
-radiation, but not 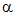 -radiation. Above 82, elements with too high a
ratio can undergo either
-radiation. Above 82, elements with too high a
ratio can undergo either 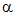 - or
- or 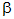 -radiation.
-radiation.
Note also, that elements with too low a ratio of neutrons:protons undergo a different type of decay in which a proton is converted into a neutron, and a positron is released:
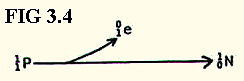
However, such nuclei are not naturally occuring, but they may be produced by nuclear reactions.
3.4. SUMMARY OF THE
PROPERTIES OF  -,
-,  -, and
-, and 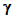 -RADIATION
-RADIATION
3.4.1. Properties
of 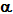 -radiation:
-radiation:
i) Nature: Fast moving helium nuclei, thus positively charged.
ii) Behaviour in an electric field: Deflected towards the negative plate.
iii) Behaviour in a magnetic field: Deflected according to Fleming's left hand rule (FIG. 3.5.):
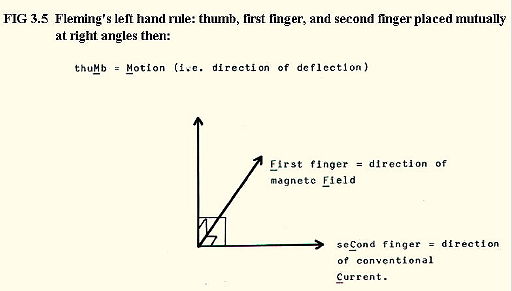
Note that the direction
of flow of the 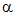 -particles = the direction of flow of
conventional current.
-particles = the direction of flow of
conventional current.
iv) Ionising
power: 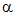 -particles have a powerful ionising effect on
any gases they pass through.
-particles have a powerful ionising effect on
any gases they pass through.
v) Penetrating
power: 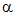 -radiation is absorbed by 7cm of air or by a
sheet of paper.
-radiation is absorbed by 7cm of air or by a
sheet of paper.
3.4.2. Properties
of 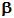 -radiation:
-radiation:
i) Nature: Fast moving electrons, thus negatively charged.
ii) Behaviour
in an electric field: Deflected towards the positive plate,
and deflected to a greater extent than 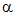 -particles owing to the low mass of an
electron.
-particles owing to the low mass of an
electron.
iii) Behaviour
in a magnetic field: Deflected according to Fleming's left
hand rule, and thus in the opposite direction to 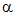 -radiation, as well as to a greater extent.
-radiation, as well as to a greater extent.
iv) Ionising
power: 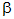 -particles are less ionising than a-pariticles
as predictable from their lower mass and lower kinetic energy.
-particles are less ionising than a-pariticles
as predictable from their lower mass and lower kinetic energy.
v) Penetrating
power: 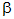 -radiation can travel a few metres through
air, and through thin sheets of metal. The denser the metal, the
thinner the sheet that can be penetrated.
-radiation can travel a few metres through
air, and through thin sheets of metal. The denser the metal, the
thinner the sheet that can be penetrated.
3.4.3. Properties
of 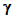 -radiation:
-radiation:
i) Nature: High frequency electromagnetic radiation.
ii) Behaviour in an electric field: Unaffected.
iii) Behaviour in a magnetic field: Unaffected.
iv) Ionising power: Weakly ionising.
v) Penetrating
power: 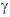 -radiation can pass through several kilometers
of air through up to 15cm of lead.
-radiation can pass through several kilometers
of air through up to 15cm of lead.
3.5. KINETICS OF
RADIOACTIVE DECAY
3.5.1. Radioactive elements decay according to first order kinetics (section 8.1.): the rate is proportional to the number of radioactive atoms present, and the half-life is constant (section 9.1.2. and table 9.1.).
In this context, half-life is the time taken for half the original number of radioactive atoms to disintegrate. During this period, the intensity of radiation will obviously fall to half its original value.
3.5.2. An equation: If you wish to remember an equation describing the rate of radioactive decay, remember this one:
..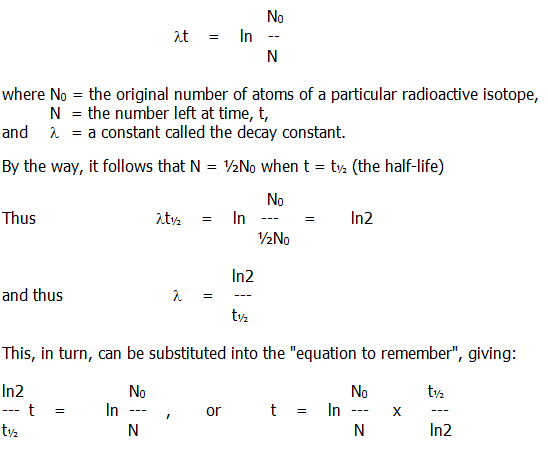
You may have the misfortune of encountering such a mathematical manoeuvre. Hopefully, your examiners will not require you to take part in one.
3.6. APPLICATIONS OF
RADIOACTIVITY
3.6.1. Carbon dating: The concentration of radioactive 14carbon dioxide in the atmosphere is assumed to have been constant throughout history (about 1 molecule in 10,000).
During their lifetime, living organisms absorb radioactive carbon, either during photosynthesis (plants) or indirectly via feeding (animals) on other living organisms. It is therefore assumed that throughout history the proportion of radioactive to non-radioactive carbon in living organisms has been constant.
When a living organism dies, it stops absorbing radioactive carbon and the radioactive carbon decays with a half-life of 5570 years. By measuring the ratio of radioactive to non-radioactive carbon in material derived from living organisms, it is therefore possible to estimate its age since death.
3.6.2. Tracers and labelling: The fate of a molecule in a living organism can be traced by labelling the molecule with a radioactive isotope. In this method, one atom in each of the molecules to be traced is replaced with a radioactive isotope.
For example, a
particular carbon atom in each molecule of a sample of glucose can be
replaced by 14carbon. (In fact, replacement is
not 100%.) If the glucose is fed to an organism, the fate of the
glucose 14carbon atom can be traced by
detecting and locating the  -radiation. This can provide
information about the types of molecule produced from the glucose, and
the location of those molecules within the organism and its cells.
-radiation. This can provide
information about the types of molecule produced from the glucose, and
the location of those molecules within the organism and its cells.
However, labelling is not exclusive to biochemistry and medicine. For example, by replacing the oxygen atoms in an ester with 18oxygen, it is possible to determine which bond is broken during ester hydrolysis (section 22.3.6.i.).
3.7. QUESTIONS
1) Account for the
different behaviour of  -,
-, 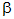 -, and
-, and 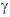 -radiation in
-radiation in
i) an electric field, ii) a magnetic field.
2) Comment on the following statements:
i) Isotopes are radioactive atoms of an element.
ii) Half-life is half the time taken for a sample of a radioactive element to decay totally.
iii) There is more
similarity between 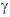 -radiation and light, than there is between
-radiation and light, than there is between 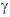 -radiation and
-radiation and  -radiation.
-radiation.
3) Fill in the missing data (indicated by question marks) in the following schemes. You will need a periodic table to identify the unamed elements.
..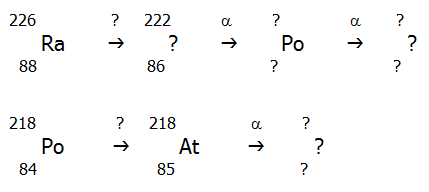
Will the final nucleus at the end of each chain be stable?
4) How valid are the assumptions on which carbon dating is based? (The 14carbon isotope is produced in the atmosphere by the bombardment of nitrogen by cosmic rays.)
5) How would you use 18oxygen to determine which bond is broken during ester hydrolysis?
Unless otherwise stated, all materials in this web version of chapter 3 are © 2007 Adrian Faiers MA (Oxon) MCIPR

What 's the connection between a dozen eggs and
a garden mole?

Answer: Not a lot, really, but see Chapter 1