Recommended by:
Top
20 UK science resources,
The Tutor Website
Recommended by:

Rated:

2010
Listed on the science,
engineering
and technology section of 
'providing you with access
to the very best Web resources for education and research, evaluated
and selected by a network of subject specialists.'
(Please note that intute closed in July 2011)
Section 1: Essentials (physical chemistry)
CHAPTER 7:
ENERGETICS
(and the first law of thermodynamics)
NB This chapter has now been
updated to improve browser compatibility.
Please
use the 'send email' link at the top right hand corner of this page to
report any problems.
QUICK SKIPS:
(Click on the 'back' arrow to get back to this quick skip section)
The First Law of Thermodynamics
Hess's Law
Hess's Law triangles
Born-Haber Cycles
7.1. CHEMICAL DELINQUENTS
7.1.1. Chapter 6: Energy, Chapter 7: Energetics - what's the difference? As we all know, energy on its own is not enough. It needs proper channelling. In chemistry, proper use of the concept of energy is called energetics, and energetics is, in turn, part of the wider topic: thermodynamics.
At first sight, thermodynamics seems to be an obsession with discovering laws. In fact, the laws turn out to be very important. In this chapter we shall encounter the first law of thermodynamics: another reason for interrupting the chemical energy tour we started in chapter 6 is that we need to discuss the first law before we encounter the second.
7.2. THE FIRST LAW OF THERMODYNAMICS
7.2.1. The first law of thermodynamics states that in a system of constant mass, energy can neither be created nor destroyed, but may be converted from one form into another.
A special development from this law is particularly useful when dealing with the enthalpy changes which accompany chemical reactions. It was formulated by a chemist called Hess.
7.3. HESS'S LAW
7.3.1. Hess's Law states that the enthalpy change accompanying a chemical reaction depends only on the initial and final states, and is independent of the route taken.
This idea is particularly useful when trying to deduce enthalpy changes for reactions where it is not possible to make the necessary measurements for direct calculation of enthalpy change.
7.3.2. For
example, it may not be possible to make the necessary
measurements for direct calculation of the enthalpy change associated
with the reaction: A g
B (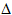 H1).
However, the enthalpy changes may be known for the reactions: A g C (
H1).
However, the enthalpy changes may be known for the reactions: A g C (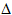 H2),
and C g B (
H2),
and C g B (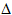 H3).
Applying Hess's Law:
H3).
Applying Hess's Law:
7.4. HESS'S LAW TRIANGLES
7.4.1. The example in section 7.3.2. can be set out diagramatically in a Hess's Law triangle. Provided the steps are carried out in a simple, logical order, the method is particularly useful in more complex cases. Using the simple example for now:
STEP 1: Write the equation for the reaction whose enthalpy change you are trying to calculate:
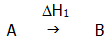
STEP 2: Use the information about other reactions (one reaction at a time) to construct an alternative route in the form of a triangle:
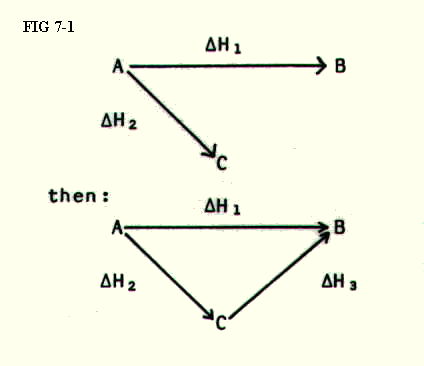
STEP 3: Apply Hess's
Law.
(Some books apply a bizarre algebraic process at this stage which bears
little relationship to Hess's Law as stated above.)
7.4.2. A more complex Hess's Law triangle. Calculate the standard enthalpy of formation of methane, given the following information:
Standard enthalpies of
combustion of:
carbon = -393.5kJmol-1
hydrogen = -285.8kJmol-1
methane = -890.4kJmol-1
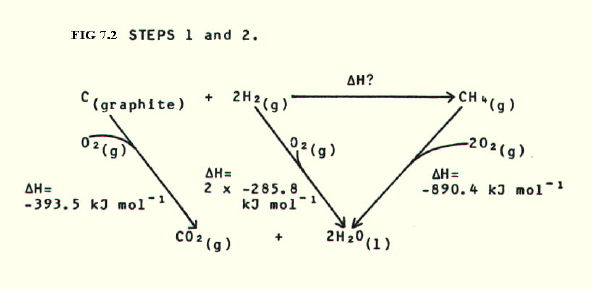
STEP 3: By Hess's Law:
 H? = (-393.5)
+ (2 x -285.8) - (-890.4)
H? = (-393.5)
+ (2 x -285.8) - (-890.4)
= -393.5 - 571.6 + 890.4
= - 74.7kJmol-1
Note that i) in the triangle, the value for enthalpy of combustion of
hydrogen is doubled because 2 moles of hydrogen are involved;
..............ii)
in the alternative route, the final step occurs in the opposite
direction from that for which the enthalpy change is given. For this
reason, the sign of the enthalpy value is changed. This is shown in
step 3 of the calculation as the minus sign in front of
the term "(-890.4)", not the minus sign in the bracket. This simply
means that a reaction which is exothermic in one direction will be
endothermic in the other direction.
7.5. BORN-HABER CYCLES
7.5.1. Born-Haber cycles could be thought of as complex Hess's Law rectangles. One major application of Born-Haber cycles is in the determination of lattice enthalpies, which cannot be obtained directly from a single experiment.
7.5.2. The lattice enthalpy of sodium chloride can be calculated by constructing an alternative route from processes for which the enthalpy changes are known. Then Hess's Law is applied:
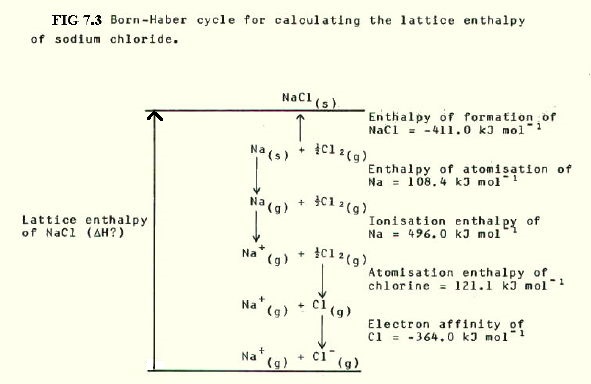
By Hess's Law:
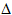 H? = -
(-411.0) + (108.4) + (496.0) + (121.1) + (-364.0)
H? = -
(-411.0) + (108.4) + (496.0) + (121.1) + (-364.0)
= 411.0 + 108.4 + 496.0 + 121.1 - 364.0
i.e Lattice enthalpy = 772.5kJmol-1
Note that just because we have constructed an alternative route, that does not mean we actually carry it out in the laboratory.
Note also that in recent years, lattice enthalpy is more often defined in terms of the enthalpy change when the lattice is formed from its gaseous ions. Although somewhat illogical, given bond enthalpies are defined in terms of bonds breaking, this is what A-level examining boards now expect and they mark as wrong values and definitions based on breaking the lattice.
7.5.3. A nice touch. Sometimes Born-Haber cycles are drawn so that the relative length of the arrows is proportional to the energy change involved. Moreover, endothermic reactions are drawn with arrows pointing up the page, and exothermic reactions are drawn with the arrows pointing down the page i.e. the position of the horizontal line on the page represents energy level - a line higher on the page represents a higher energy level.
Fortunately, examiners usually give you the diagram on which to fill in details. Nevertheless, one of these luxurious Born Haber cycles is shown in FIG 7.4. and it is good to practice drawing one or two, at least with relative energy levels represented diagramatically, if not drawn 'to scale'.
7.6. QUESTIONS (enthalpies are given in kJmol-1)
1) The standard enthalpies of formation are given below for several compounds:
carbon dioxide = -393.7
water = -241.8
methane = -74.8
ethane = -84.6
propane = -103.8
Using the above data:
a) Give values for the standard enthalpies of combustion of carbon (graphite) and hydrogen.
b) Using Hess's Law triangles, calculate values for the standard enthalpies of combustion of methane, ethane, and propane.
c) Make calculations to decide which of the following will produce the greatest amount of heat: burning the gas from i) a cylinder containing 50g of methane, or ii) a cylinder containing 50g of ethane, or iii) a cylinder containing 50g of propane.
2) Some mean bond enthalpies and some atomisation enthalpies are given below
Mean bond enthalpies:
C-H 413
C-C 346
C=C 611
Atomisation enthalpies:
Carbon (graphite) 715
Hydrogen 218
i) From the above data calculate a value for the enthalpy of formation of buta-1,3-diene.
ii) Why is the use of mean bond enthalpies particularly inaccurate in this case?
iii) Will the value calculated in part a) be higher or lower than the real value? Explain why.
3) i) Construct a Born-Haber cycle and calculate the lattice enthalpy of magnesium oxide from the following enthalpy changes:
Enthalpy of formation
of magnesium oxide = -602
Atomisation energy of magnesium = 149
Sum of magnesium's first two ionisation energies = 2240
Atomisation energy of oxygen = 249
Sum of oxygen's first two electron affinities = 649.
ii) Which values in the Born-Haber cycle for beryllium oxide would you expect to be different? Give your reasons saying whether the enthalpy values in question would be greater or smaller.
iii) Using Born-Haber cycles (without numerical values) explain why magnesium forms MgCl2 rather than MgCl or MgCl3. Choose your wording carefully; be careful not to imply that magnesium behaves in the way that it does because of favourable or unfavourable Born-Haber cycles.
Unless otherwise stated, all materials in this web version of chapter 7 are © 2007 Adrian Faiers MA (Oxon) MCIPR

What 's the connection between a dozen eggs and
a garden mole?

Answer: Not a lot, really, but see Chapter 1