Recommended by:

Top 20 UK science resources,
The Tutor Website
Recommended by:

Rated:

2010
Listed on the science, engineering and technology section of

'providing you with access to the very best Web resources for education and research, evaluated and selected by a network of subject specialists.'
(Please note that intute closed in July 2011)
Section 1: Essentials (physical chemistry)
CHAPTER 8: KINETICS
AND EQUILIBRIUM
(an introduction)
NB This chapter has now been
updated to improve browser compatibility.
Please
use the 'send email' link at the top right hand corner of this page to
report any problems.
QUICK SKIPS:
(Click on the 'back' arrow to get back to this quick skip section)
Confusion
Order of reaction
Rate expression
The Law of Mass
Action
Equilibrium
expression
The partial
pressure of a gas
Table summarising
the differing effects of various factors on rate, rate constant,
equilbrium and equilibrium constant
Le Chatelier's
Principle
8.1. CONFUSION
8.1.1. Confusion between kinetics theory and equilibrium theory is one of the classic mistakes at this level - and beyond. The muddle may stem from the fact that equilibrium is dynamic, but that is probably a generous excuse. It is more likely to originate at relatively superficial levels. For example, rate expressions look a bit like equilibrium expressions.
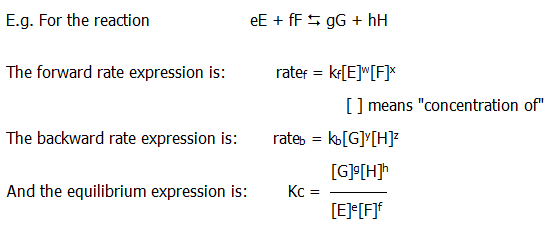
The expressions are similar, but different: i) The equilibrium expression is a ratio of concentrations whereas the rate expressions are not.
...........................................................ii) Rate constants are represented by a small k whereas equilibrium constants are represented by a capital K.
..........................................................iii) There is a difference in the powers to which the concentrations are raised in the two types of expression. In the rate expression the powers may or may not be equal to the molar quantities in the stoichiometric equation for the reaction. In contrast, the powers in the equilibrium expression do equal these molar quantities.
....................Thus in the forward rate expression above:
....................w may or may not equal e
....................x may or may not equal f
....................and so on.
..........................................................iv) In rate expressions the powers are referred to as orders (of magnitude) whereas in equilibrium expressions they are not. Thus in the forward rate expression above, w is known as the order with respect to E, x is the order with respect to F, and so on. w + x is the overall order of the forward reaction.
It is easy to lose sight of the fact that "order" merely refers to the orders of magnitude in the rate expression. This is especially so when terms such as first order, second order, etc. are used. A second order reaction is simply one in which the overall order (total of the powers, or orders of magnitude) is 2.
..........................................................v) Further experiments are needed to establish the orders in the rate expressions. In contrast, the powers in the equilibrium expression are are based on the same experimental data as those underlying the stoichiometric equation.
Two simple methods for determining order are:
a) Direct determination of the relationship between rate and concentration - initial rate and initial concentration are usually easiest to examine.
b) The half-life method.
We shall examine these methods in more detail in chapter 9.
..........................................................vi) Obviously the fundamental difference between rate and equilibrium expressions is that they describe different things! What they do describe, we shall decide in the next two sections.
8.2. RATE EXPRESSION
8.2.1. Before we can decide what is described by rate expressions, we should be clear what is meant by the rate of a reaction.
The rate of a reaction is the rate at which reactants are used up or products are formed. It is usually measured in moldm-3 of one reactant used, or one product formed, per second, i.e. moldm-3s-1.
Rate expressions describe the relationship between rate of reaction and the concentrations of reactants. So too, does the law of mass action:
8.2.2. The law of mass action states that at constant temperature, the rate of a reaction is directly proportional to the active mass (effectively the concentration) of the reacting substances.
It is only partly correct.
It is true that under given conditions of temperature, pressure, and concentration of reactants, the rate of a particular reaction will always be the same. It is even true that at constant temperature, the rate may depend directly on the concentration of one or more of the reactants. However, as implied in our discussion of order (section 8.1.), this is not always the case.
8.2.3. The rate expression describes more accurately the relationship between rate of reaction and concentrations of the reactants. It does so for a particular temperature, the temperature for which the rate constant is quoted.
In the example of E reacting with F (section 8.1.) it might be found by experiment that the forward reaction is zero order with respect to E and first order with respect to F. This would mean that the rate was directly proportional to the concentration of only one reactant, F.
8.3. EQUILIBRIUM EXPRESSION
8.3.1. To decide what equilibrium expressions describe, we shall continue with the example reaction between E and F (section 8.1.). When E and F are mixed, they react to produce G and H. Thus the concentrations of E and F decrease, and the rate of forward reaction decreases as time goes on (assuming it depends on the concentration of at least one reactant, E or F).
The forward reaction also produces increasing concentrations of G and H. G and H then react to re-form E and F. Moreover, the rate of this backward reaction increases as time goes on (assuming it depends on the concentration of at least G or H).
Eventually the rate of the backward reaction equals the rate of the forward reaction. This state is known as equilibrium. At equilibrium, both forward and backward reactions continue to take place, but at the same rates. Hence there is no nett reaction in either direction. The concentrations of all the reactants and products stay constant.
8.3.2. The equilibrium expression describes the composition of the equilibrium mixture. It does so in terms of a ratio between product and reactant concentrations. For a particular reaction, this ratio is shown to be a constant at the temperature for which it is quoted. It is known as the equilibrium constant, Kc.
In the case of gaseous reactions, partial pressures may be used in the expression instead of concentrations. In this case the equilibrium constant is represented by Kp.
8.3.3. The partial pressure of a gas in a mixture of gases is equal to:
Total pressure x mole fraction of the gas.
8.3.4. The mole fraction of a gas in a mixture of gases is equal to:
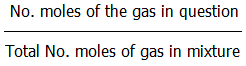
8.4. Kc vs. k: A SIMPLE
RELATIONSHIP
Now we are clear about the differences between rate and equilibrium expressions, it is useful to see how they are related. Continuing with the example reaction between E and F, we shall look at the special case when:
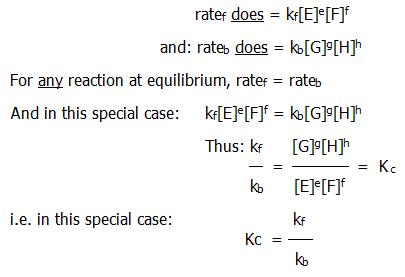
Given the dynamic nature of equilibrium some relationship between equilibrium constant and the ratio of forward and backward rate constants might have been expected. It is not always this simple, but a relationship always exists and it helps to define the space between kinetics and equilibrium.
8.5 MORE CONFUSION
The space between kinetics and equilibrium needs to be especially clear when considering changes in reaction conditions.
Tables such as TABLE 8.1. are dangerous. They tempt students to remember rather than understand. Nevertheless, the differing effects of temperature, pressure, concentration, and catalysts, on rate, rate constant, composition of equilibrium mixtures, and equilibrium constants are so confusing that it is a good idea to have them summarised clearly.
By the end of chapters 9 and 10, you should be able to predict for yourself the behaviour set out in the table. Even the law describing the changes in equilibrium (section 8.6.) should be considered only as a rule of thumb.
|
TABLE. 8.1. Table summarising
the effects of changes in temperature, pressure, concentration and
catalysts on: rate, rate constant, composition of equilibrium mixtures
and equilibrium constants. |
||||
|
Effect on: Factor |
rate |
Rate constant (k) |
Composition of equilibrium
mixture |
Equilibrium constant (K) |
|
|
|
k=Ae-Eact/RT ( |
Reaction
proceeds in endothermic direction until ratio of concs. = new value of K |
Where Y is a constant ( |
|
|
|
No change |
Reaction
proceeds to oppose pressure change until equilibrium is
restored. |
No change |
|
|
|
No change |
Reaction
proceeds to oppose conc. Change until ratio of concs. = K |
No change |
|
catalyst |
+ve
cat -ve
cat |
New pathway with new k |
No change |
No change |
8.6. LE CHATELIER'S PRINCIPLE
8.6.1. Despite the statement at the end of the last section, Le Chatelier's principle is a perfectly valid law based on experiment. It summarises a great deal of empirical observation and allows predictions to be made. We could not ask much more from a chemical law except that Le Chatelier's principle does not really improve our understanding of the observations.
8.6.2. Le Chatelier's principle states that when the conditions of a system at equilibrium are changed, the system responds in a way which opposes the change.
For example, if the temperature of a system at equilibrium is increased, the system responds in a way which tends to reduce the temperature i.e. equilibrium moves in favour of the endothermic direction.
Another problem with this principle is that it can makes the system sound a bit human (teliology), but we shall see in chapter 10 how the opposing responses are quite involuntary!
8.7. QUESTIONS
1) For the reaction in aqueous solution between ethanoic acid and ethanol producing ethyl ethanoate:
i) Write the stoichiometric equation.
ii) Write three possible forward rate expressions, assuming the reaction is second order.
iii) What would be the units of the rate constant in each case?
iv) Write the equilibrium expression.
v) If the reaction is carried out in dilute aqueous solution, write another ratio of concentrations that would remain constant even if more ethanoic acid were added to the system at equilibrium;
vi) Describe the difference between rate and rate constant.
vii) Describe the difference between equilibrium and equilibrium constant.
viii) Explain how the composition of the equilibrium mixture remains constant despite the fact that reactions continue to occur.
ix) With reference to the effects on rate and equilibrium, describe what will happen to the ethyl ethanoate concentration when the following changes are made to the system (assuming the reaction is at equilibrium before each change):
a) a small amount of pure ethanoic acid is added;
b) a small amount of water is added;
c) a large amount of water is added;
d) atmospheric pressure increases;
e) a few drops of concentrated sulphuric acid (catalyst) are added;
f) a moderate amount of concentrated sulphuric acid is added;
g) the temperature is increased.
Unless otherwise stated, all materials in this web version of chapter 8 are © 2007 Adrian Faiers MA (Oxon) MCIPR

What 's the connection between a dozen eggs and
a garden mole?

Answer: Not a lot, really, but see Chapter 1