Recommended by:
Top
20 UK science resources,
The Tutor Website
Recommended by:

Rated:

2010
Listed on the science,
engineering
and technology section of 
'providing you with access
to the very best Web resources for education and research, evaluated
and selected by a network of subject specialists.'
(Please note that intute closed in July 2011)
Section 1: Essentials (physical chemistry)
CHAPTER 10: MORE ON
EQUILIBRIUM
NB This chapter has now been
updated to improve browser compatibility.
Please
use the 'send email' link at the top right hand corner of this page to
report any problems.
10.1. SOLUBILITY
This section will set the tone for the chapter: there is a lot of terminology to come to terms with. The terminology is intended to help you make predictions by enabling you to describe equilibria clearly and concisely.
10.1.1. Saturated solutions and solubility: When excess salt is added to water it dissolves until the rate of dissolution is equalled by the rate of precipitation i.e. until equilibrium is reached.
E.g............................. CaF2(s)
+ water....  .... Ca2+(aq)
+ 2F-(aq)
.... Ca2+(aq)
+ 2F-(aq)
or more generally:........... AxBy
+ water....  ....
xAy+(aq) + yBx-(aq)
....
xAy+(aq) + yBx-(aq)
At this point, the solution is said to be saturated. Moreover, the concentration of salt in a saturated solution is its solubility. The solubility of calcium fluoride is 2.31 x 10-4moldm-3 compared with that for sodium chloride of 5.42moldm-3. Solubilities are also often measured in gdm-3, and even in g per 100 cm3!
Summarising, "solubility" is a measure of how soluble a substance is, and "saturated" describes solutions which have dissolved as much of a substance as possible i.e. solutions in which the "solubility" has been reached.
10.1.2. Solubility product: The equilibrium expression for the general example above of a salt in equilibrium with its saturated solution is given by:
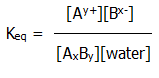
However, the concentrations of the solid and of the water (~ 55M) are effectively constant, so the following ionic product is also a constant:
Ksp = [Ay+][Bx-]
It is known as the solubility product, and is usefully applied to sparingly soluble salts (i.e. salts with solubilities up to about 10-3moldm-3).
The solubility product is easily calculated from the solubility (though remember it may be necessary to convert the solubility into moldm-3 first. Taking the example of calcium fluoride:
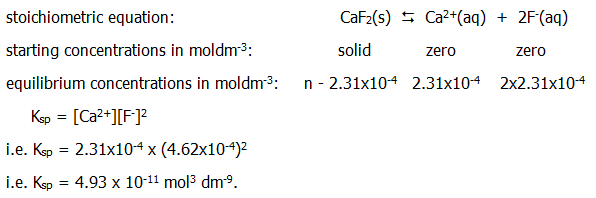
(Note the units. Note also that Ksp = [Ca2+][F-]2 not [Ca2+][2F-]2, whatever that might mean, or [Ca2+]2[F-]2, or any other similar attempt to get an extra 2 in there somewhere.)
The above format is a useful way of simply laying out equilibrium calculations, which can otherwise become very complicated. Note that one mole of dissolved salt, produces three moles of dissolved ions.
Summarising, the "solubility product" is the product of the maximum concentrations which can exist in solution, of the ions of an electrolyte. It is a function of the equilibrium constant for the dissolution of the electrolyte.
10.1.3. The common ion effect: Solubility products are, of course, constant at a given temperature. They therefore give a measure of the maximum ionic product at a given temperature, for a particular pair of ions in solution.
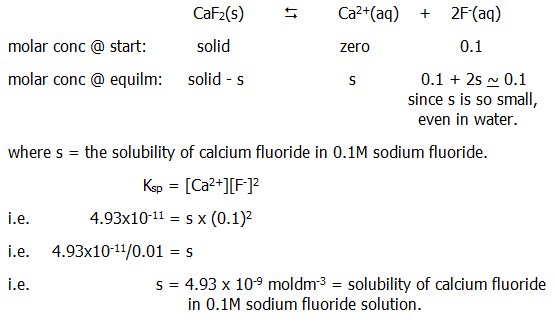
From this, we can *predict that a salt will be less soluble in a solution already containing one of its ions than it is in water. More than this, we can calculate the actual solubility, for example in 0.1M sodium fluoride:
Moreover, we can also *predict that addition of a common ion to a solution of calcium fluoride will cause precipitation of the salt if the solubility product is exceeded. Or, we can *predict that addition to a solution containing one of the ions of a solution containing the other, will cause precipitation if the solubility product is exceeded. Again we can also make calculations. For example we can calculate the minimum concentration of fluoride ions needed to precipitate calcium fluoride from a 0.1M solution of calcium nitrate:
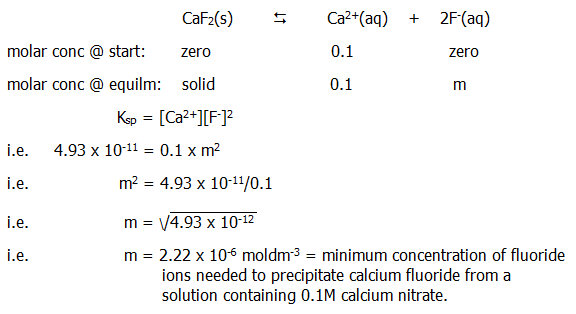
Say this was achieved by adding 0.01M ammonium fluoride solution. It would be necessary to add 2.22x10-6 moles of ammonimum fluoride to each dm3 of calcium nitrate solution, i.e. 2.22x10-6/0.01 dm3 i.e 2.22x10-4dm3 i.e. 0.222 cm3 i.e. not very much.
(* The above predictions could also be made by applying Le Chatelier's principle from section 8.6.)
Summarising, The "common ion effect" is the effect that the presence of an ion from another source has on the solubility of a sparingly soluble electrolyte containing the same ion.
10.2. ACID/BASE EQUILIBRIA
10.2.1. Definitions: there are two currently used definitions of acids and bases:
i) Brønsted-Lowry:...... acid = proton donor
.....................................base = proton acceptor
e.g. ......HB(aq)... +...
H2O(l)...... ...... H3O+(aq)...... +......
B-(aq)
...... H3O+(aq)...... +......
B-(aq)
............acid.............. base.................. acid........................ base
ii) *Lewis:................... acid = electron pair acceptor
....................................base = electron pair donor
Note that the Lewis definition also covers the example given to illustrate the Brønsted-Lowry definition. This is because Brønsted-Lowry acids donate protons by accepting electron pairs, and Brønsted-Lowry bases accept protons by donating electron pairs.
However, the Lewis definition recognises a more generally applicable characteristic of the behaviour than that recognised by the Brønsted-Lowry definition. It therefore includes a great deal of other behaviour which is not covered by the Brønsted-Lowry definition. (E.g. sections 20.6. and 20.9.). The Lewis definition can be regarded as an electrostatic attraction model of how Brønsted-Lowry acids/bases (and other many reactants) work.
In this sense, the Lewis definition is better because it unifies a greater number of cases. Combined with the electrostatic approach (section 14.4.2.v.) this is particularly useful. It is useful because it rationalises the basis for making predictions. *However, the Lewis definition is not generally included in A-level specifications.
Moreover, it is sometimes helpful to look at more limited areas. Rationalisation does not always equal simplification. Ironically, we have already seen this with the concept of energy: it is often easier to go back to a consideration of the electrostatic forces involved (sections 6.1.1. and 14.4.2.v.). It is a question of balance between describing everything in one sentence and describing everything in realistic sections.
In short, the more unifying the theory, the further it becomes removed from what we actually notice in our observations. We might end up with an answer to "Life, the Universe and Everything" like that in the third book of the "Hitchiker's Guide To The Galaxy" series. The answer in this humorous science fiction book, is a single number, 42.
The Brønsted-Lowry definition provides a unified approach to fewer cases than the Lewis definition, but it does seem to pick on an aspect of acid/base behaviour which seems most characteristic of the substances which we most clearly recognise as acids (particularly) and bases. It plainly describes what was recognised as special about those substances which were first classified in this way, substances like hydrochloric acid and sulphuric acid (acids), or sodium hydroxide and calcium carbonate (bases).
The Brønsted-Lowry definition is limited, but it does move on from earlier definitions of acids and bases, which were even more limited by simple observations, and therefore limited in the extent to which they enabled predictions to be made. Such definitions included:
An acid is a substance which produces hydrogen gas when it reacts with electropositive metals or, slightly less limited, it is a substance which produces an excess of hydrogen ions over hydroxide ions in aqueous solution.
Conversely, a substance which produces an excess of hydroxide ions over hydrogen ions in solution is called an alkali.
You should become very familiar with the common acids, hydrochloric, sulfuric (VI), nitric (V) and ethanoic. (Note that because sulfuric (VI) acid is more commonly encountered than sulfuric (IV) acid, the VI is often omitted when naming it. Similarly, nitric acid refers to nitric (V) acid rather than nitric (III) acid.) You should also be familiar with the common alkalis, sodium hydroxide, potassium hydroxide and ammonia solution. You should not have to think when it comes to recalling their formulae and understanding what ions they produce in solution.
You should also be aware that when an acid reacts with an alkali, the essential reaction, neutralisation, is simply:
............................H+ ....+ ....OH- ......→ ......H2O
However, you should understand the reactions (and therefore be able to write equations) of acids with a range of bases reactions, including carbonates and metal oxides (NB click on each word for two separate links), to produce salts plus water. Note that the reaction with carbonates is one of the two commonly encountered effervescent reactions of acids. What makes the reaction effervescent and what is the other commonly encountered effervescent reaction of acids? With alkalis, the salt ions remain in solution and are therefore spectator ions, hence the simplified ionic equation above.
In this section of the book we shall be talking about Brønsted-Lowry acids and bases. Unifying theory is essential to have, but not always helpful to apply.
10.2.2. Question: When is an acid not an acid? Answer: When it's a base.
This may seem odd, but an acid is defined by its behaviour. Ethanoic acid is generally regarded as a weak acid. This is because in the most common setting for acids, aqueous solution, some of the ethanoic acid molecules donate protons to water molecules. Ethanoic acid is an acid because it donates protons; it is weak because only some of its molecules donate protons, i.e. it is only partially dissociated:
HEt(aq)...... +......
H2O(l)...... ......
H3O+(aq)...... +......
Et-(aq)
......
H3O+(aq)...... +......
Et-(aq)
acid.....................
base..................
acid........................
base
However, if hydrogen chloride is dissolved in pure ethanoic acid, the following equilibrium is set up:
HCl(et)...... +......
HEt(l)...... ......
H2Et+(et)...... +......
Cl-(et)
......
H2Et+(et)...... +......
Cl-(et)
acid....................
base....... .........
acid......................
base
In this situation, ethanoic acid acts as a base, a fact we could have predicted, because we know hydrogen chloride is a stronger acid than ethanoic acid. In water hydrogen chloride is essentially completely dissociated:
HCl(g)...... +......
H2O(l)...... ......
H3O+(aq)...... + Cl-......(aq)
......
H3O+(aq)...... + Cl-......(aq)
The importance of water as the typical environment for acids and bases is seen in the definitions of weak and strong acids and bases:
Weak acids and weak bases are those which are partially dissociated in water.
Strong acids and strong bases are those which are completely dissociated in water.
Water itself
acts both as an acid and a base, and is said to be an amphiprotic
solvent. Even when pure, it shows both acidic and basic behaviour.
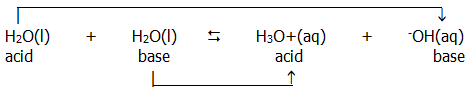
10.2.3. Degree of
dissociation and dissociation constants: The strength of a
weak acid or base, or other weak electrolyte, can be measured in terms
of its degree of dissociation. The degree of dissociation (a) of a weak electrolyte, is the
percentage of one mole of the electrolyte that is dissociated at a
particular dilution. (An electrolyte is a substance
which undergoes dissociation into ions in aqueous solution.)

Ka is the acid's dissociation constant. For ethanoic acid, a weak acid, the value is 1.7 x 10-5. A similar dissociation constant, Kb, can be described for weak bases (study question 1).
10.2.4. (Ostwald's) dilution law: Consider the case of a particular concentration of weak acid dissolved in water:
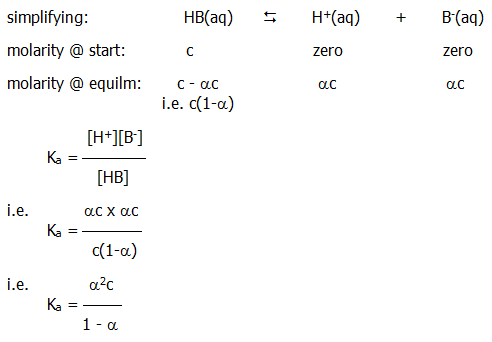
*But, for a weak acid a is very much less than 1, therefore 1 - a ~ 1,
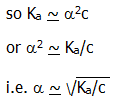
This is a
semi-mathematical statement of (Ostwald's) dilution law
which in words observes that: the degree of dissociation of a weak
electrolyte ( <
approx. 10-2) is inversely proportional to the
square root of its concentration (or directly proportional to its
dilution, the volume containing one mole).
<
approx. 10-2) is inversely proportional to the
square root of its concentration (or directly proportional to its
dilution, the volume containing one mole).
Remember, however, that the dissociation constant remains unchanged unless the temperature changes. Note also that a similar procedure can be applied to weak bases (question 10.1.), since it is generally applicable to weak electrolytes.
* This is another of those steps which recognises the insignificance of small numbers in certain combinations with large numbers. See also the solubility of calcium fluoride in sodium fluoride (section 10.1.3.). Such steps are essential in simplifying many equilibrium calculations.
10.2.5. Dissociation constant of water: From the equation at the end of section 10.2.2., it can be seen that water contains equal concentrations of hydrogen and hydroxide ions. The liquid is neutral. When an acid is added, the hydrogen ion concentration, [H3O+], exceeds the hydroxide ion concentration, [-OH], and the solution is acidic. When a base is added, the hydroxide ion concentration exceeds the hydrogen ion concentration, and the solution is alkaline.
Applying equilibrium law to the same equation at the end of 10.2.2. would give:
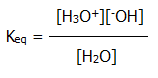
But the degree of dissociation of water is very low, and the concentration of water is therefore again effectively constant. The following ionic product is therefore also constant:
....Kw =
[H3O+][-OH]
= the dissociation constant for,
...................................or
ionic product of, water.
At 25°C,............. Kw = 10-14mol2dm-6
Moreover, since. [H+] = [-OH]:
then:.................. Kw = [H+]2
so:................... [H+] = /10-14
i.e.................... [H+] = 10-7M, which also = [-OH].
Thus, at 25°C, aqueous solutions which contain a hydrogen ion concentration (or hydroxide ion concentration) of 10-7M, are neutral. Addition of acids or alkalis to water can be seen as special cases of the common ion effect (section 10.1.3.).
Note also that since the dissociation of water is an endothermic process, we can predict from Le Chatelier's principle (section 8.6.) that increasing the temperature will increase the concentrations of hydrogen and hydroxide ions, and that decreasing temperature will decrease their concentrations. However, neither increasing, nor decreasing the temperature of water affects the balance between hydrogen and hydroxide ions, i.e. the water stays neutral.
10.2.6. pH: The concept of pH has been introduced into chemistry because it is considered inconvenient to deal with values for [H+] like 3.86 x 10-5M. pH replaces such expressions with a simple number. It is given by:
pH = -log10[H+]
Thus the pH of an acidic solution containing 3.86 x 10-5M hydrogen ions would be 4.41. However, it could be argued that although this makes the numbers more convenient to handle, it does make it less obvious that, for example, the hydrogen ion concentration in a solution with a pH of 6 is 10x greater than it is in a solution with a pH of 7. Always remember that pH values are negative logarithms to the base 10, and that a change of one unit represents a ten fold difference in hydrogen ion concentration.
Nevertheless, pH is generally regarded as a useful concept. For example, although a measure of hydrogen ion concentration, it is also possible to work out the pH of an alkaline solution. This can be done because it is possible to calculate the hydrogen ion concentration from the ionic product of water (section 10.2.5.).
Thus, for an alkaline solution containing 3.86 x 10-5M hydroxide ions:
..........................Kw = [H+][-OH]
therefore......... [H+] = Kw/[-OH]
thus at 25°C.... [H+] = 10-14/3.86 x 10-5
i.e.................. [H+] = 2.59 x 10-10
thus................... pH = -log(2.59 x 10-10)
i.e..................... pH = 9.59.
The pH of a neutral
solution at 25°C is clearly 7, i.e. -log 10-7
(section 10.2.5.). It can also be deduced that at 25°C acidic solutions
will have a pH < 7 and alkaline solutions will have a pH
> 7 (question 10.5.). At 25°C the normal range of pH is from
0  14:
14:
1 molar strong acid:...... pH = -log 1 = 0
neutral solution:........... pH = -log 10-7 = 7
1 molar strong alkali:.... pH = -log 10-14/1 = 14
More concentrated solutions of strong acids will produce negative pH values and more concentrated solutions of strong alkalis wil produce pH values greater than 14.
However, at high concentrations even strong acids and bases may not be completely dissociated, so the values become inaccurate. Moreover, the use of concentration in equilibrium (and rate) expressions is an approximation. Strictly speaking, the so-called active mass (or activity) should be used. At high concentrations, concentration is not a satisfactory substitute for active mass.
10.2.7. Acid/base titrations: The titration of a base with an acid (or vice versa) provides useful information about the acid and the base.
FIG.10.1. shows the
variation in pH as a base (BOH) is added to a given volume (V) of acid
(HA) of equal concentration. This would be achieved by measuring, say,
25cm3 of the acid into a conical flask and
adding alkali slowly from a burette. After each addition the pH of the
solution would be measured using a pH meter (section 11.1.2.). The
results would then be plotted.
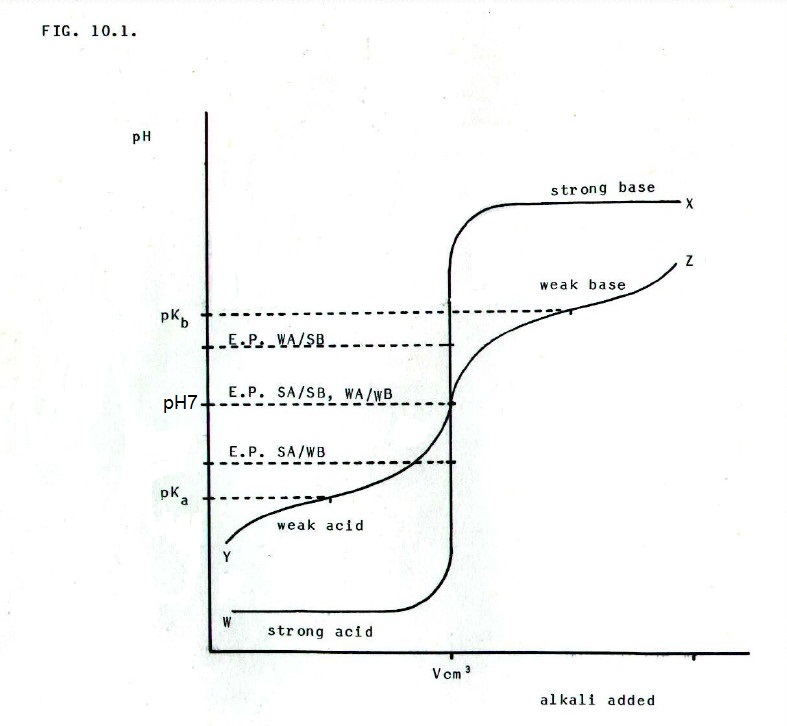
Curve WX = curve obtained for
titration of a strong acid with a strong base
...............=
SA/SB (e.g. hydrochloric acid with sodium hydroxide).
Curve WZ = curve
obtained for titration of a strong acid with a weak base
...............=
SA/WB (e.g. hydrochloric acid with ammonia solution).
Curve YX = curve
obtained for titration of a weak acid with a strong base
..............=
WA/SB (e.g. ethanoic acid with sodium hydroxide).
Curve YZ = curve
obtained for titration of a weak acid with a weak base
..............=
WA/WB (e.g. ethanoic acid with ammonia solution).
EP = the equivalence point, which is the pH when equivalent amounts of acid and alkali have been mixed. Equivalent amounts are amounts which would, if fully dissociated, produce the same number of moles of hydrogen or hydroxide ions. In titrations, the equivalence point is more commonly referred to as the end point of the titration.
In the titration of an acid with a base of equal strength (not necessarily the same concentration), the solution at the equivalence point is a solution of the salt. If the acid and base differ in strength, the solution at the equivalence point can still be regarded as a solution of the salt, but the equivalent of a hydrolysed salt solution (section 10.2.10.). Of course, the history of the two solutions is different, though the end results are the same.
Clearly, titrations such as this can be used to determine whether an acid or base is strong or weak. Moreover, they can be used to determine pKa and pKb values (-logKa and -logKb respectively). Thus the antilogs give the values of the respective dissociation constants, Ka and Kb.
Note that the pKa of an acid is the pH at which dissociated and undissociated forms are present in equal concentrations. Similarly, the pKb of a base is the pH at which its dissociated and undissociated forms are present in equal concentrations. Work these conclusions through.
From FIG. 10.1., note also that addition of acid or alkali to solutions with pH values close to the pKa of an acid or the pKb of a base, has a small effect on the pH of the solution. This enables such solutions to be used as buffers (section 10.2.9.).
10.2.8. Indicators: Acid/base titrations can also be used to determine the concentration of a particular solution of acid or base using standard solutions of bases or acids respectively. However, it is not always convenient to use a pH meter to determine the end-point. Indicators are more useful.
An indicator is a substance which changes colour according to the pH. Most are weak acids in which the associated acid is one colour and its conjugate base is another colour:

It is generally
reckoned that to see a colour change, the concentration must change by
a factor of 10. When [H+] is
increased by a factor of 10 ( a decrease
by 1 pH unit) the ratio of [In-]/[HIn] will
decrease by a factor of 10 and the predominant colour will be that of
HIn. Work this through from the expression for KIn.
a decrease
by 1 pH unit) the ratio of [In-]/[HIn] will
decrease by a factor of 10 and the predominant colour will be that of
HIn. Work this through from the expression for KIn.
When [H+]
is decreased by a factor of 10 ( increase by 1 pH unit) the [In-]/[HIn] ratio will
increase by a factor of 10 and the predominant colour will be that of In-.
Work this through.
increase by 1 pH unit) the [In-]/[HIn] ratio will
increase by a factor of 10 and the predominant colour will be that of In-.
Work this through.
It follows from this that the working range of an indicator is approximately equal to the pKIn ± 1 pH unit.
This is useful information when choosing an indicator to determine the end-point of an acid/base titration. From FIG. 10.1. it can be seen that the choice of indicator depends on the relative strengths of the acid and base being titrated. The working range of the indicator should lie well within the point of rapid pH change during the titration, the vertical part of the graph.
Some suitable choices for different titrations are summarised in TABLE 10.1.
|
TABLE 10.1 Indicator choices
for acid base titrations |
||||
|
Titration |
Suitable indicator |
pKIn |
Working range |
Colour change |
|
SA/SB |
Almost any,
e.g. bromothymol blue |
7.0 |
6.0 g 7.6 |
Yellow / blue |
|
SA/WB |
Screened
methyl orange |
3.7 |
3.2 g 4.2 |
Purple / green |
|
WA/SB |
phenolpthalein |
9.3 |
8.2 g 10 |
Colourless / pink |
|
WA/WB |
The pH change
is so gradual at the end point, that colour
changes are difficult to observe; other methods such as pH meter or
conductance measurements must be used (sections 11.12.4 and 11.15.4). |
|||
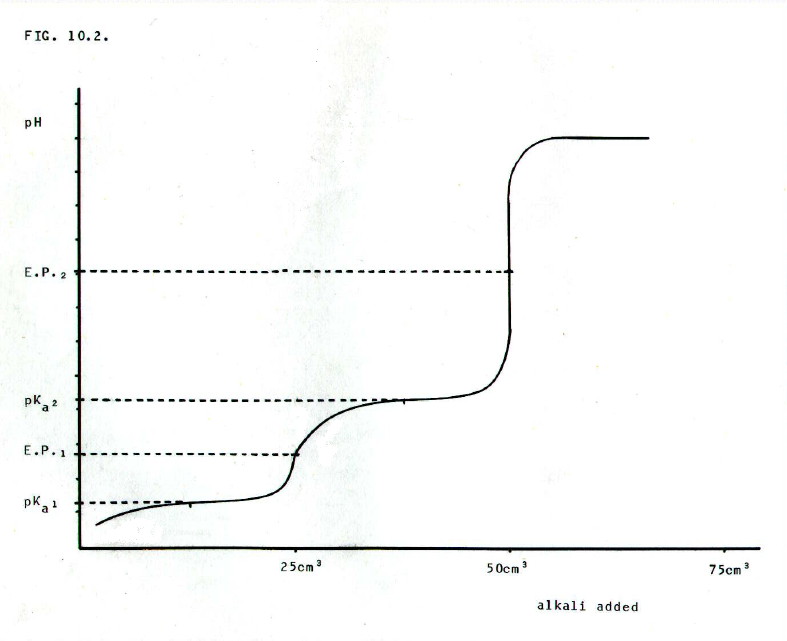
Moreover, some acids are able to donate more than one proton, and some bases are able to accept more than one proton. In titrations to determine concentration, the indicator must be carefully chosen in these cases.
For example,
ethanedioic acid is a weak dibasic acid. Loss of the first proton
occurs at a lower pH than loss of the second proton. There are two pKa
values and two equivalence points. Depending on the choice of
indicator, either the first or the second end point may be detected, as
shown in FIG. 10.2.
10.2.9 Buffers:
A buffer is a solution which maintains a fairly constant pH even when
moderate quantities of strong acids or bases are added.
i) Acid buffers are, not surprisingly, those which maintain an acid pH. They contain a weak acid plus its conjugate base, preferably in equal concentrations, and can be made in one of two ways:
either by mixing a weak acid with one of its soluble salts, preferably in equimolar proportions,
E.g. 50ml 0.1M HEt +
50ml 0.1M NaEt  100ml 0.05M acid
buffer
100ml 0.05M acid
buffer
or by titrating a weak acid with a strong base, ideally until the pH = pKa (section 10.2.8. and FIG. 10.1.)
E.g. 50ml 0.2M HEt +
50ml 0.1M NaOH  100ml 0.05M same buffer
100ml 0.05M same buffer
ii) Alkaline buffers are those which maintain an alkaline pH. They contain a weak base plus its conjugate acid, preferably in equal concentrations, and can be made in one of two ways:
either by mixing a weak base with one of its soluble salts, preferably in equimolar proportions,
E.g. 50ml 0.1M NH3
+ 50ml 0.1M NH4Cl  100ml 0.05M alkaline buffer
100ml 0.05M alkaline buffer
or by titrating a weak base with a strong acid, ideally until the pH = pKb. (section 10.2.8. and FIG. 10.1.)
E.g. 50ml 0.2M NH3
+ 50ml 0.1M HCl  100ml 0.05M same buffer.
100ml 0.05M same buffer.
iii) Mode of action acid buffers: In the acid buffer described above, the following (simplified) relationships exist:
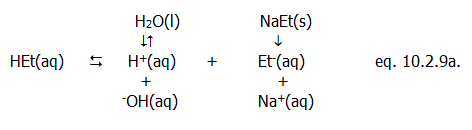
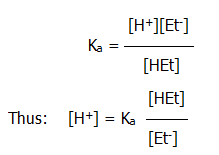
But, the acid is only partly dissociated, especially in the presence of the ethanoate ions from the salt,
Thus:.. [HEt] ~ original [acid]
and..... [Et-] ~ original [salt]
since all the ethanoate ions have effectively come from the salt, given the partial dissociation of the acid.

Applying Le Chatelier's principle, and referring to eq. 10.2.9a. above, it can be predicted that:
i) If acid is added, the added hydrogen ions combine with ethanoate ions from the salt and are effectively removed from solution. The hydrogen ion concentration and pH therefore stay roughly the same.
For example, if 1cm3 of 0.1M hydrochloric acid is added to the 100ml of acid buffer described above, the new hydrogen ion is given by:
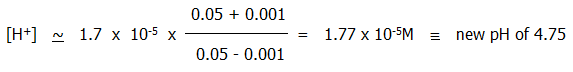
By contrast, adding the same amount of 0.1M hydrochloric acid to 100ml water would reduce the pH from 7 to 3.
ii) If alkali is added, the added hydroxide ions combine with hydrogen ions, but these are effectively replaced by further dissociation of undissociated acid (eq. 10.2.9a.). The hydrogen ion concentration and pH therefore stay roughly the same.
For example, if 1 cm3 of 0.1M sodium hydroxide is added to the 100ml of acid buffer described above, the new hydrogen ion concentration is given by:
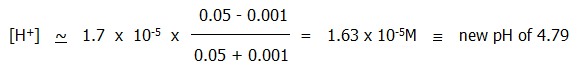
By contrast, adding the same amount of 0.1M sodium hydroxide to 100ml water would increase the pH from 7 to 11.
iv) Effectiveness of buffers: It can be seen from these calculations that the most effective buffers start with equivalent concentrations of acid and salt (conjugate base) as stated at the start of this section. Moreover, within obvious limitations, the higher the concentration of the buffer, the more effective it is.
v) The applications of buffers are varied. They are used to calibrate pH meters and indicators (study question 10.9)
They are also used when a precise pH is needed during the course of a reaction, especially when that reaction itself produces hydrogen or hydroxide ions (Table 22.1. note ii).
Control of pH during reactions is particularly important in biochemical reactions, because biological macromolecules are profoundly affected by pH (e.g. section 25.2.5.). Enzymes, in particular, are active only within precise pH limits. For this reason, buffers are also extremely important in medicine. Injected drugs, for example, must be dissolved in buffers in so as not to disturb the blood pH.
Moreover, naturally occurring buffers exist in living organisms for the same reasons. As well as buffers such as the hydrogen carbonate system in blood, perhaps the most important biological buffers are proteins themselves, since they have weak acidic and basic groups, as well as salt groups on their surface (section 25.2.).
10.2.10. Salt hydrolysis: i) The salt of a strong acid and a weak base (such as ammonium chloride) undergoes salt hydrolysis according to the following (simplified) relationships:
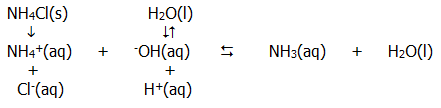
From a knowledge of equilibria we can predict that the ammonium ions from the completely dissociated salt remove hydroxide ions from solution. Further dissociation of water will occur, but the overall result is an excess of hydrogen ions giving an acidic solution.
The overall reaction can therefore be summarised as:
....NH4+(aq).... + H2O(l)...... ...... NH3(aq).... +....
-OH(aq)
...... NH3(aq).... +....
-OH(aq)
for which, given the effectively constant value of [H2O], a hydrolysis constant can be described:
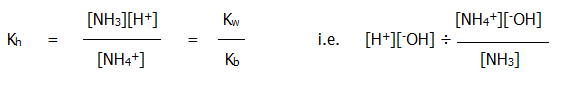
ii) The ions produced in solution by a weak acid and a strong base (such as sodium ethanoate) are related in the following (simplified) way:
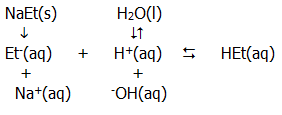
Thus, the ethanoate ions from the salt will combine with hydrogen ions. Further dissociation of water will occur, but an excess of hydroxide ions over hydrogen ions will remain, giving an alkaline solution.
In this case the following relationships can be derived:
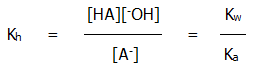
iii) If the salt of a weak acid and a weak base (such as ammonium ethanoate) is dissolved in water, both ions are hydrolysed, the effects roughly cancel each other out, and an approximately neutral solution is produced. When the salt of a strong acid and a strong base (such as sodium chloride) is dissolved in water, no hydrolysis occurs and a neutral solution is produced.
10.3. EQUILIBRIUM AND ACTIVATION ENERGY
The relationship between equilibrium and rate is firmly established by considering the effect of an increase in temperature on forward rate, backward rate, and equilibrium - something merely to read, understand, and file away.
FIG.10.3. shows the
energy profile (i) of reaction A + B  C + D, and
the energy distribution (ii) of the molecules in the reaction mixture
at two temperatures.
C + D, and
the energy distribution (ii) of the molecules in the reaction mixture
at two temperatures.
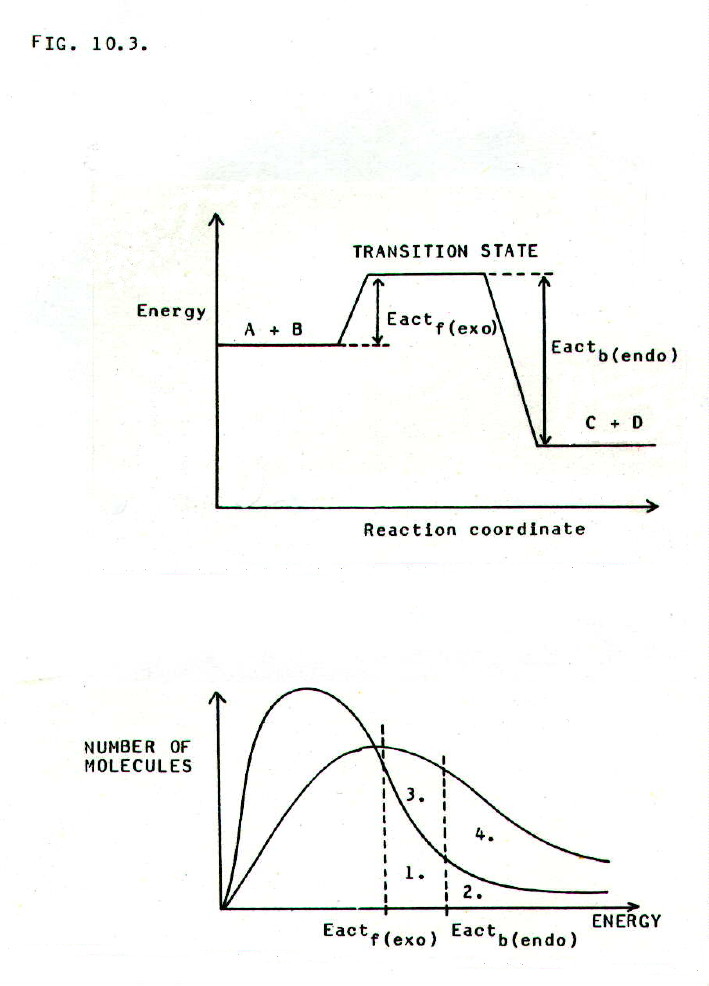
It can be seen that for both the
forward (exothermic) and backward (endothermic) reactions, increasing
the temperature increases the proportion of molecules with equal to, or
greater than, the activation energy. It therefore follows that
increasing the temperature increases the frequency of favourable
collisions in both directions, and therefore increases the rate in both
directions, endothermic and exothermic.
However, graph (ii) shows a significant difference between the two directions:
For the forward (endothermic) direction the number of molecules with sufficient energy for reaction increases as shown by the increase in relevant areas on the graph from 1&2, to 1&2 + 3&4.
For the backward (endothermic) direction the number of molecules with sufficient energy for reaction increases as shown by the increase in relevant areas on the graph from 2, to 2 + 4.
The increase is
proportionately greater in the endothermic direction. Thus, when the
temperature is increased, the increase in frequency of favourable
collisions is proportionately greater in the endothermic direction. In
other words if the system
A + B  C + D is at equilibrium, and the temperature
is increased, the rate will increase in both directions, but the rate
in the endothermic direction will increase more than the rate in the
exothermic direction.
C + D is at equilibrium, and the temperature
is increased, the rate will increase in both directions, but the rate
in the endothermic direction will increase more than the rate in the
exothermic direction.
There will therefore be nett reaction in the endothermic (backward) direction. However, this will cause the concentrations of C&D to fall, and the endothermic (backward) rate will therfore fall as time goes on. Similarly, after the temperature increase, the concentrations of A&B will rise, and the exothermic (forward) rate will therefore rise as time goes on.
Eventually, the falling endothermic (backward) rate will be equalled by the rising exothermic (forward) rate and a new state of equilibrium will have been reached at the new, higher, temperature.
However, not only will the composition of the equilibrium mixture have changed in favour of the endothermic direction, but also the ratio [C][D]/[A][B], i.e. the equilibrium constant will have changed in favour of the endothermic direction.
This is consistent with the observations in section 8.4. In simple cases
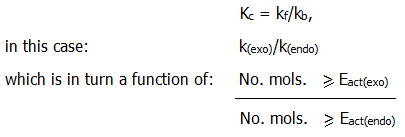
We therefore have a more detailed description of an effect predicted by Le Chatelier's rule of thumb (section 8.6.)
10.4. QUESTIONS
1) Write a parallel
text based on sections 10.2.3. and 10.2.4. for a weak base such as NH3(aq)
+ H2O(l)  NH4+(aq)
+ -OH(aq)
NH4+(aq)
+ -OH(aq)
2) To which of the following does Ostwald's dilution law apply: sodium chloride, ammonia solution, silver chloride? Explain your reasoning.
3) Discuss the extent to which addition of acid or alkali to water is a special case of the common ion effect.
4) Derive an expression showing the relationship between pKw, and pH and pOH, where p means -log. Then derive a simplified expression for the pH at 25°C.
5) Show that acidic solutions have a pH < 7 at 25°C and that alkaline solutions have a pH > 7 at the same temperature.
6) From the expression for Ka, for an acid HB, show that when pH = pKa, the concentrations of dissociated and undissociated acid are equal.
7) 25ml of a sodium hydroxide solution are measured into a conical flask using a pipette. 27ml of 0.01M sulphuric acid are required to reach the end point. What is the concentration of the sodium hydroxide solution, and what indicator would you have used in the titration?
8) Describe the mode of action of the buffer described in section 10.2.9.ii. by writing a text parallel to that in section 10.2.9.iii.
9) You are provided with: i) universal indicator which shows a range of colours over the whole pH range ii) 14 buffer solutions of known pH increasing in whole pH units from 1 to 14 iii) 15 test tubes iv) three aqueous solutions of unknown pH. Describe precisely how you would determine the approximate pH of the three unknown solutions.
10) Derive the relationship described in section 10.2.10 between Kh for the salt of a weak acid/strong base, and Ka for the acid, and Kw.
11) Use reasoning like that in section 10.3. to predict the action of a positive catalyst on the composition and equilibrium constant of an exothermic equilibrium reaction. Is this what you expected? Explain.
Unless otherwise stated, all materials in this web version of chapter 10 are © 2007 Adrian Faiers MA (Oxon) MCIPR

What 's the connection between a dozen eggs and
a garden mole?

Answer: Not a lot, really, but see Chapter 1