Recommended by:

Top 20 UK science resources,
The Tutor Website
Recommended by:

Rated:

2010
Listed on the science, engineering and technology section of

'providing you with access to the very best Web resources for education and research, evaluated and selected by a network of subject specialists.'
(Please note that intute closed in July 2011)
Section 3: Organic
CHAPTER 21: PREDICTING
REACTIONS [ I ]
(Hydrocarbons and simple derivatives)
NB This chapter has now been
updated to improve browser compatibility.
Please
use the 'send email' link at the top right hand corner of this page to
report any problems.
QUICK SKIPS:
(Click on the 'back' arrow to get back to this quick skip section)
Alkanes
Alkenes
Alkynes
Inductive and Mesomeric
effects
Benzene and other
aromatic hydrocarbons
Haloalkanes
21.1. INTRODUCTION
In chapters 21 and 22 we shall look at the reactions of different types of organic molecule. We shall attempt to predict main reaction types from structure and then, for each type of molecule, we shall briefly summarise reactions which do not easily fall into the 12 types described in the last chapter.
If you are unfamiliar with the types of molecule considered in these chapters, then chapter 27 (Nomenclature) should help. Look up each type of molecule as you consider its reactions.
Some indication of reaction conditions will be given. It is ridiculous to learn a whole list of conditions: if they are needed for laboratory procedures they can be looked up.
However, reaction conditions also give an indication of the ease with which a reaction occurs. They should certainly be absorbed at a sub-conscious level to help you acquire a feel for relative reactivities. Once you have such a feel, you will be able to predict reaction conditions as accurately as can reasonably be expected. If your examiners require more, they are wasting your time.
21.2.1. Predictions: Alkanes have no regions of either exposed nuclear charge or high electron density and are therefore unaffected by either nucleophiles or electrophiles.
Moreover, there are no polarised bonds, so reactions occur homolytically, when they occur at all. In addition, bonds are strong so reactive free radicals are needed to make alkanes react.
Finally, there are no multiple bonds, so addition is not possible. Nor is elimination favoured because this would involve simultaneous attack on hydrogen atoms attached to two adjacent carbon atoms - an unlikely event. The result of attack by free radicals on an alkane is therefore substitution i.e. the nett reaction is homolytic substitution, via mechanism 1 (FIG. 20.1.).
21.2.2. Homolytic substitution in alkanes: examples of attacking free radicals:
| Table 21.1. Examples of free radical substitution in alkanes (see section 20.3 for mechanism) | |||
| Radical | Reagent | Conditions | Product(s) |
 Cl Cl |
chlorine |
gaseous and UV
light |
chloroalkane, dichloroalkanes etc |
 Br Br |
bromine | gaseous/heat/UV | bromoalkane, dibromoalkanes etc |
 SO2.OH SO2.OH |
fuming sulphuric acid | heat | alkanesulphonic acid (salts = detergents) |
 NO2 NO2 |
concentrated nitric acid | heat/gas phase | nitroalkanes (mixture due to fission etc.) |
21.2.3. Other reactions: Two other homolytic reactions undergone by alkanes are cracking and combustion. These are not chain reactions, but like homolytic substitution, the conditions needed for reaction are extreme, i.e. high temperature:
i) cracking: The bonds break rather randomly in cracking reactions, producing a mixture of saturated and unsaturated hydrocarbons.
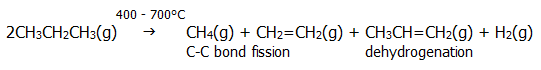
ii) combustion:
CH4(g).... +....
2O2(g).... ....
CO2(g)....
+.... 2H2O(g)
....
CO2(g)....
+.... 2H2O(g)
2C2H6(g).... +....
7O2(g).... ....
4CO2(g)....
+.... 6H2O(g)
....
4CO2(g)....
+.... 6H2O(g)
Note that combustion is an oxidation reaction. Alkanes may also undergo "autoxidation", by a free radical chain mechanism. This can be initiated by light, or an "initiator". Typical initiators are substances which produce free radicals, sometimes at higher temperatures or in the presence of light. Autoxidation is a bad term because it implies that the process takes place in the absence of any other reactant. In fact, the oxidising agent is atmospheric oxygen.
21.3.1. Predictions i) The double bond in alkenes is a region of high electron density which therefore attracts electrophiles. Moreover, the molecule is unsaturated and the attack results in addition. i.e. nett reaction is electrophilic addition (by mechanisms 8 and 9).
However, the implied connection between unsaturation and addition begs the question, "What favours saturation over unsaturation?"
The answer is
illustrated in the equation below. The electrons involved in the bonds
resulting from addition (bonds iii, iv and v), are held more tightly
than they were before addition occured (bonds i and ii); electrons are
pulled away from the double bond and the Br-Br bond, into single bonds
where they are held more tightly.
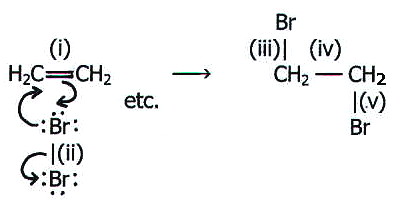
The relative tightness with which the electrons are held before and
after addition can be understood in the following way. The pair of
electrons in the p
orbitals of double bond (i) are not particularly close to the two
carbon nuclei. They become more strongly held in the two s bonds (iii and v) since these
are directly inbetween the carbon and bromine nuclei.
Moreover, the two electrons in bond (ii) between two large bromine atoms, become more tightly held in bonds (iii) and (v), where the smaller size of the carbon atoms makes the bonding electrons closer to the nuclei.
The changes are reflected by (not explained by) the energy changes involved in the reaction.
21.3.2. Electrophilic addition to alkenes: examples of attacking electrophiles
21.3.3. Predictions ii) The above reactions occur in the absence of conditions which produce free radicals. In conditions where free radicals are present (see section 20.12.1.) addition may occur via a homolytic mechanism, i.e. homolytic addition via mechanism 6 (FIG. 20.1.).
Reactants which may add homolytically include: *Br-H (not HCl or HI), RS*-H, Cl3C*-Cl, and Cl3C*-H. * indicates the part of the molecule which forms the initial attacking radical.
Polymerisation by a homolytic addition mechanism has already been discussed in section 20.12.2.
21.3.4. Another example of homolytic addition, but not a chain reaction, is the reduction of alkenes by hydrogen gas in the presence of a metal catalyst such as platinum, or finely powdered nickel.
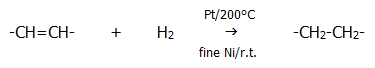
The binding sites on the nickel for hydrogen atoms are slightly further apart than the length of the H-H bond. This tends to split the hydrogen into reactive atoms. Hydrogenation of double bonds is an important process in the manufacture of some margarines. Saturated fats tend to be more solid than unsaturated oils, though the health implications are well known.
21.3.5. Other reactions:
i) Oxidation:
a) Combustion:
CH2=CH2(g)... +...
3O2(g)....... .......
2CO2(g)...
+... 2H2O(g)
.......
2CO2(g)...
+... 2H2O(g)
b) With acidified potassium manganate (VII) solution:
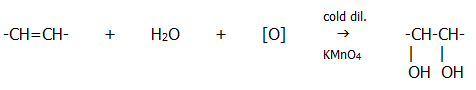
Note that this reaction involves a readily observable change. The purple colour of the manganate (VII) disappears, and brown manganese (IV) oxide is precipitated.
c) With ozone:
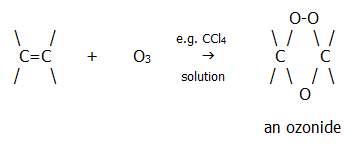
Ozonides are explosive and are not isolated. However, hydrolysis of the ozonide is a useful reaction. It produces carbonyl compounds (provided a reducing agent such as zinc dust and ethanoic acid is present to prevent oxidation of the carbonyls by the hydrogen peroxide):
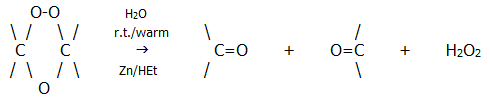
The overall reaction with ozone, followed by hydrolysis, is known as ozonolysis and its usefulness lies in its power as an analytical tool:
analysis of the resultant carbonyls gives information about the structure of the parent alkene. For example, what alkene would produce a mixture of propanone and ethanal on ozonolysis?
21.4.1. Predictions i) The arguments are similar to those for alkenes. The triple bond in alkynes is a region of high electron density which therefore attracts electrophiles. Moreover, alkynes are unsaturated and attack results in addition. The nett reaction is therefore electrophilic addition via mechanisms 8 and 9 (FIG. 20.1.).
Note that, as discussed in section 20.15, alkynes are often less reactive than alkenes to electrophiles, despite their higher electron density.
Note also, that after addition to a triple bond, there is still a double bond. This may undergo further electrophilic addition. However, reactivity may be less than expected if the first addition to the triple bond has introduced, say, a halogen atom into the molecule:
A halogen atom attached to a doubly bonded carbon atom has a negative inductive effect (section 21.5.). This reduces the electron density in the double bond and makes it less susceptible to electrophilic attack than a double bond in a simple alkene. Moreover, further addition will be directed as predicted by Markownikoff's rule (section 20.14.1.).
21.4.2. Examples of electrophilic addition to alkynes
The electrophiles which add to alkynes are largely the same as those which add to alkenes (table 21.2.), and in the absence of free radicals, the main product is predicted by Markownikoff's rule. However, remember that alkynes are generally less reactive than alkenes and:
(i) Bromine water does not react.
(ii) the addition of halogens or halogen halides requires a halogen carrier catalyst such as FeBr3. Alternatively, UV light enables the reaction to proceed via a homolytic mechanism. However, under these conditions, the reaction with chlorine may be explosive, producing carbon and hydrogen chloride.
(iii) addition of water
under acid conditions requires mercury (II) sulphate to further catlyse
the process. The method used is bubbling the alkene into hot dilute
sulphuric acid containing the catalyst. The "enol" so produced is
unstable and rapidly undergoes rearrangement to form a carbonyl
compound. For example:
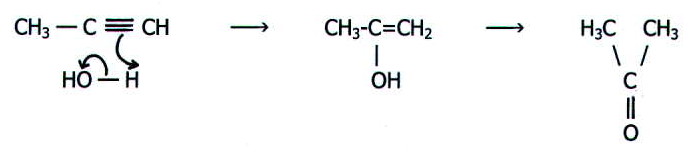
The reaction is useful in the synthesis of a large range of organic
compounds, especially when it is considered that carbon itself may be
the starting point, via calcium(II) dicarbide!
....................................
2000°C
CaO(s).. +.... 3C(s)........... ..........
CaC2(s)....
+.... CO(g)
..........
CaC2(s)....
+.... CO(g)
CaC2(s)..+..2H2O(l)........ .......
Ca(OH)2(s)..
+...CH
.......
Ca(OH)2(s)..
+...CH CH(g)
CH(g)
21.4.3. Predictions ii) Apart from electrophilic addition there is another fascinating property of alkynes. Electrons in an sp1 orbital are closer to the nucleus than those in an sp2 orbital, and even closer than those in an sp3 orbital. Under certain conditions, a hydrogen next to a triple bond can actually be removed as a proton and the C-H bonding electron pair accomodated in the carbon atom's sp1 orbital. Thus a carbanion is formed and the alkyne can be regarded as having slight acidic properties (section 21.4.4.).
21.4.4. Acidic properties. The acidic properties are shown in two ways:
i) The amide ion is a strong enough base to remove the acidic hydrogen. The reagent is sodium dissolved in liquid ammonia.
2NH3(l)... +...
2Na(s)....... ......
2Na+ -:NH2(am).... +....
H2(g)
......
2Na+ -:NH2(am).... +....
H2(g)
-C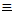 C-H(g)..+... -NH2(am).......
C-H(g)..+... -NH2(am)....... ......
-C
......
-C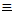 C-(am).... +....
NH3(g)
C-(am).... +....
NH3(g)
Sodium alkynides are extremely useful for the synthesis of other alkynes because the alkynide ion is a powerful nucleophile in reaction with haloalkanes (section 21.7.2.).
(ii) Also, alkynes with terminal hydrogen atoms form silver and copper(I) salts when treated with diammine complex ions of the metals. The formation of characteristic precipitates makes the reactions useful tests for 1-alkynes:
RC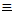 CH(g)
+ Cu(NH3)2+(aq)
+ -OH(aq)...
CH(g)
+ Cu(NH3)2+(aq)
+ -OH(aq)... ...
RC
...
RC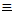 CCu(s) + H2O(l) + NH3(aq)
CCu(s) + H2O(l) + NH3(aq)
......................................................................red
ppt.
RC CH(g)
+ Ag(NH3)2+(aq)
+ -OH(aq)...
CH(g)
+ Ag(NH3)2+(aq)
+ -OH(aq)... ...
RC
...
RC CAg(s) + H2O(l) + NH3(aq)
CAg(s) + H2O(l) + NH3(aq)
....................................................................white
ppt.
21.4.5. Other reactions
i) Oxidation: Like alkanes and alkenes, alkynes undergo various oxidation reactions, not least autoxidation and combustion.
E.g. Combustion: 2CH CH(g).. +..
5O2(g)...
CH(g).. +..
5O2(g)... ...
4CO2(g)..
+.. 2H2O(g)
...
4CO2(g)..
+.. 2H2O(g)
21.5.
INDUCTIVE AND MESOMERIC EFFECTS
21.5.1. Introduction: In section 21.4.1. a new concept was slipped into the text with out explanation. What is a negative inductive effect? For that matter, what is a positive inductive effect? Briefly, inductive effects, positive or negative, are little more than polarised bonds seen with a different journalistic bent. It is important to realise that even scientific language depends on the attitude of the observer.
Inductive effects exist
in 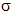 -bonds
and also in
-bonds
and also in  -bonds,
but in the former case
they do not involve delocalisation. Polarisations which do involve
delocalisation via
-bonds,
but in the former case
they do not involve delocalisation. Polarisations which do involve
delocalisation via  bonding systems and p-orbitals are known as mesomeric or conjugative
effects.
bonding systems and p-orbitals are known as mesomeric or conjugative
effects.
In fact, mesomeric and conjugative effects are little more than delocalisation seen with a different journalistic bent. They do not even involve polarisation in all circumstances.
Two further points on language: First, the different jounalistic bent described above is not totally artificial. It is useful for describing particular situations because it saves clumsy explanations. Good scientists would not make good Sun reporters, though they might do well on The Independent.
Second, inductive and mesomeric effects are often talked about as "occurring". This does not mean that they occur on any time scale. The negative inductive effect of a halogen atom does not suddenly happen in a haloalkane; it is there all the time.
21.5.2. Inductive effects exist where (occur where) two atoms or groups which differ in electronegativity are bonded.
A more electronegative
atom or group exerts a negative inductive (-I) effect, "pulling"
electrons towards itself and acquiring partial negative charge (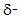 ).
).
For example, chlorine
and oxygen exert -I effects in the molecules shown here:
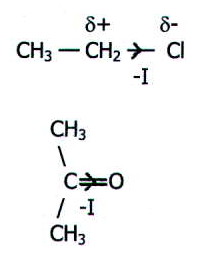
The most important groups to exert an electron pushing, or +I, effect
are alkyl groups. This is largely a characteristic of the large number
electropositive hydrogen atoms within alkyl groups.
21.5.3. Mesomeric or conjugative effects exist:
i) where 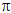 -bonding systems would otherwise
be next to each other - separated by one single bond, or
-bonding systems would otherwise
be next to each other - separated by one single bond, or
ii) where electrons in
p-orbitals would otherwise be next to 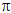 -bonding
systems - separated by one single bond.
-bonding
systems - separated by one single bond.
The term conjugation is
often reserved for situations where the polarity of the effect is not
relevant, eg in buta-1,3-diene (section 4.8.8.) The double bonds which
appear in the simple bonding diagram (FIG. 4.13.) are conjugated and
there is no polarisation. However, in phenylethene, it is more relevant
to think of the ethene group exerting a positive mesomeric (+M) effect
on the benzene ring. The 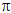 -bonding
systems are conjugated, but in this case there is polarisation:
-bonding
systems are conjugated, but in this case there is polarisation:
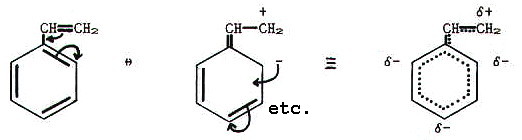
Another way of describing the situation is to say that electrons from
the alkene double bond are delocalised into the benzene ring.
In phenylethanal, the
carbonyl group is considered as exerting a negative mesomeric (-M)
effect on the benzene ring. Electrons are delocalised out of the ring
onto the electronegative oxygen atom:
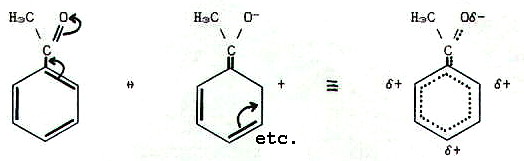
Thus doubly bonded electronegative elements exert negative mesomeric
effects as well as negative inductive effects. In other cases, the
polarity of mesomeric effects is determined by the relative electron
densities of the  -bonding
systems which overlap i.e. electrons delocalise from regions of higher
electron density to regions of lower electron density, as in the case
of phenylethene described above.
-bonding
systems which overlap i.e. electrons delocalise from regions of higher
electron density to regions of lower electron density, as in the case
of phenylethene described above.
21.5.4. Positive or negative? In the last section we described a positive mesomeric effect in phenylethene; the ethene group exerts a positive mesomeric effect on the benzene ring. However, it would appear just as valid to say that the benzene ring exerts a negative mesomeric effect on the carbonyl group. Which is correct?
Sometimes, either can be correct. Take phenylethene: If you are considering the reactions of the ethene group, it is probably most useful to think in terms of the benzene ring exerting a -M effect on the ethene group. If you are considering the reactions of the benzene ring, it is probably most useful to consider how the +M effect of the ethene group affects the benzene reactions.
Alternatively, using the other form of language, "electrons from the double bond are delocalised into the benzene ring", covers both situations.
In other cases when using the mesomeric terminology, one description certainly is better than the other. Thus in phenylethanal it would be artificial to describe the benzene ring exerting a positive mesomeric effect and "pushing" electrons onto the electronegative oxygen atom.
Similar arguments apply to inductive effects.
21.6.
BENZENE AND OTHER AROMATIC HYDROCARBONS
21.6.1.
Predictions. The high electron density of the benzene
ring's  -bonding system makes it
susceptible to attack by electrophiles. However, despite being
unsaturated, benzene does not undergo addition as a result of such
attack.
-bonding system makes it
susceptible to attack by electrophiles. However, despite being
unsaturated, benzene does not undergo addition as a result of such
attack.
Addition would involve
a concentration of the electron density within the benzene ring by
breaking the aromatic delocalisation:
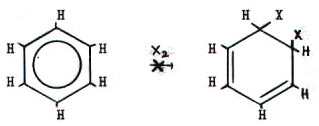
Thus substsitution of a hydrogen by the attacking
group is favoured, since this restores delocalisation. The overall
reaction is therefore elctrophilic substitution via
mechanism 5 (FIG. 20.1.).
21.6.2. Electrophilic substitution in the benzene ring: some examples.
| Table 21.3. Examples of electrophiles which react with benzene. | |||
| Electrophile | reagent | conditions | main organic products |
| NO2+ | conc. nitric dissolved in conc. sulphuric | 55°C  100°C  reflux 48hrs  |
nitrobenzene 1,3-dinitrobenzene 1,3,5-trinitrobenzene |
| *SO3 | conc. sulphuric acid | 80°C | Benzenesulphonic acid |
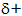 ..... ..... 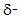 Cl-Cl--AlCl3 Br-Br--FeBr3 R-Hal--AlCl3 |
chlorine  bromine  haloalkane  |
AlCl3, FeBr3 etc, catalyst (or iron filings g FeBr3) (Lewis acids) |  chlorobenzene chlorobenzene bromobenzene bromobenzene alkylbenzene, but difficult to stop
at mono substituted stage alkylbenzene, but difficult to stop
at mono substituted stage |
| ..* RCO-Cl--AlCl3 |
acid chloride | Lewis acid halogen carrier, as above. | phenylketone (mono!) can be reduced to corresp. alkyly deriv. eg by Zn amalgam/HCl. |
| + CH3=CH2 |
ethene | acid to protonate alkene, (HCl/H3PO4), plus Lewis acid | ethylbenzene
(mono!) Industrially: +Zn/600°C  |
| * marks the electron deficient centre in the electrophile (if not already obvious). | |||
21.6.3. Effects of the rest of the molecule. The table shows that it is easy to stop some reactions at the mono substitution stage, but difficult to stop others. This highlights an interesting piece of theory. Electron pushing and electron withdrawing groups attached to the benzene ring affect its reaction with electrophiles in two ways:
i) they make it either more reactive (activate) by increasing electron density in the ring, or less reactive (deactivate) by decreasing the electron density in the ring;
ii) they direct electrophiles to particular positions in the ring by changing the distribution of electron density i.e. by making it more concentrated around particular carbon atoms. Electrophiles are more likely to attack in these positions.
Looking at this in more detail emphasises a point about models. It is easiest to see how these effects come about by using a model of the benzene ring which looks less like the real thing than the model which shows delocalisation. Models are only models and, as previously stated, different models serve different functions.
21.6.4. Positive
mesomeric effects e.g. NH2 group.
i) Electron pushing groups activate the ring
towards electrophilic attack because the electron density is increased.
ii) They are also 2,4,6-directing because the electron density is increased more in the 2,4 and 6 positions.
Other groups which similarly activate and 2,4,6-direct are: -OH, -OR, -NHR, -NR2, -C6H5, etc.
21.6.5. Negative
mesomeric effects e.g. -COR.
i) Electron withdrawing groups deactivate the ring
towards electrophilic attack because the electron density is decreased.
ii) They are also 3,5-directing, because the electron density is reduced less in the 3 and 5 positions positions.
Other groups which
similarly deactivate and 3,5-direct are: -COOH, -NO2,
-C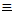 N, -SO3H,
etc.
N, -SO3H,
etc.
21.6.6. Inductive and combined effects
The same effects can be
brought about by inductive mechanisms. The most important groups to
exert a positive inductive effect on the ring are alkyl groups:
Groups which exert a negative inductive effect are -CF3,
-CCl3 etc.
Obviously, +I effects
activate and 2,4,6-direct, and -I effects deactivate and 3,5-direct.
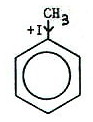
However, some groups exert both mesomeric and inductive effects. In the
case of the nitro group this is simple: it exerts both a -M and a -I
effect.
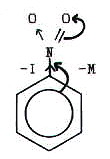
However, the halogens, for example, exert a +M effect, but a -I effect.
As a result, halogen atoms 2,4,6-direct, but weakly deactivate the
benzene ring.
21.6.7. Summary of effects of benzene ring substituents on
electrophilic attack.
| Table 21.4. The effects of substituents on electrophilic substitution into the benzene ring. | |
| 2,4,6-directing | 3,5-directing |
| strongly
activating -NH2, -NHR, -NR2, -OH |
|
| moderately
activating -NHCOCH3, -NHCOR, -OCH3, -OR |
|
| weakly
activating -CH3, -C2H5, -R, -C6H5 |
|
| weakly
deactivating -Cl, -Br, -I |
|
| moderately
deactivating -SO3H, -COOH, -CHO, -C 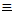 N N |
|
| strongly
deactivating -NO2, -CF3, -CCl3 |
|
These effects are important to consider when:
i) Predicting the main products when benzene and its derivatives react;
ii) predicting reaction conditions;
iii) choosing methods to synthesise benzene derivatives
....and so on.
21.6.8. Other reactions
i) Addition. Extreme conditions are needed to enable addition to the benzene ring, in particular, the presence of free radicals eg:
ii) Oxidation.
a) Benzene undergoes combustion in air to produce Carbon dioxide and water.
2C6H6.... +....
15O2........ ......
12CO2....
+.... 6H2O
......
12CO2....
+.... 6H2O
However, combustion of aromatic compounds is incomplete, giving a smokey black flame due to production of carbon. In qualitative analysis, the smokey flame is often taken as an initial indication that an aromatic, or highly unsaturated, compound is present.
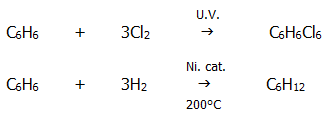
b) The controlled
catalytic oxidation of methyl benzene by air (diluted with nitrogen to
prevent further oxidation to benzenecarboxylic acid) is used to make
benzenecarbaldehyde.
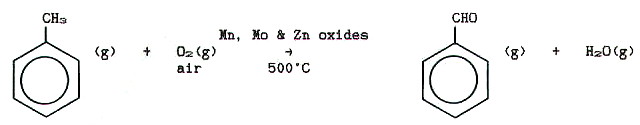
c) A reaction of some industrial importance is the controlled catalytic
oxidation of benzene by air to butenedioic (maleic) anhydride used in
making varnishes and lacquers.
d) Benzene reacts with ozone at room temperature:
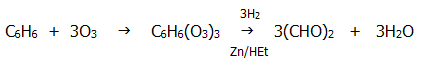
e) Any side
chain connected to the benzene ring via a carbon atom is
oxidised by refluxing with acid or alkaline potassium manganate(VII),
or acidified potassium dichromate(VI), or dilute nitric(V) acid etc.
Whatever the side chain, it is always oxidised to -COOH. E.g.
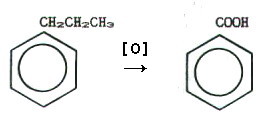
21.6.9. Methylbenzene (Toluene)
Methylbenzene is an important compound whose reactions differ from benzene in two important ways:
i) The methyl group activates the ring towards electrophiles, and 2,4,6-directs (section 21.6.2)
ii) The side chain undergoes its own reactions such as the oxidation described in section 21.6.8.iie above, as well as reactions typical of alkanes described in section 21.1.
21.7.1. In this section we shall break the pattern of dealing completely with each prediction in turn. This is because the different types of reaction are in such close competition, they must be considered together.
21.7.2. Predictions i) Halogen atoms are electronegative and attract the bonding electrons towards themselves and away from the atoms to which they are bonded. In haloalkanes, this exposes nuclear charge on the functional carbon atom:
...........................................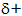 ...
... 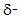
.........................................RCH2-Hal
As a result, the carbon atom is susceptible to attack by nucleophiles. Moreover, the electronegative halogen atom is able to acquire total control of the bonding electrons. This occurs as a pair of electrons from the nucleophile forms a new bond with the functional carbon. Attack therefore results in substitution and the overall reaction is nucleophilic substitution via mechanisms 2 and 3 (FIG. 20.1.).
The reactivity of the haloalkanes to nucleophilic substitution decreases in the order I > Br > Cl (>F). This order of reactivity is paralleled by the order of C-Hal bond lengths and bond strengths (section 22.2.2.).
21.7.3. Predictions ii) But there is another possibility. The halogen is a good "leaving group" for the reason stated above, but it may not always be substituted. Sometimes, the attacking species pulls a hydrogen atom off a carbon next to the functional carbon atom. In such a case, it is acting as a base rather than a nucleophile. The overall reaction is therefore elimination via mechanisms 10 and 11 (FIG. 20.1.).
21.7.4. Elimination
vs. substitution:The balance between elimination and
nucleophilic substitution is affected by three factors:
.....................................i)
the attacked species,
....................................ii)
the attacking species, and
...................................iii)
the "external" conditions.
i) Attacked species: The more branching that exists around the functional carbon atom in the attacked species, the more difficult it is for an attacking base/nucleophile to reach this exposed nuclear charge.
Thus elimination becomes less likely in the series:
.....3°.........................
2°............................
1°
..R......................... R
...\.......................... \
R-C-Hal........ >........ CH-Hal........ >........ R-CH2-Hal
.../.......................... /
..R......................... R
ii) Attacking species: The stronger the attacking base/nucleophile, the more likely it is that a hydrogen will be removed from the attacked species. Both nucleophiles and bases act by having a pair of electrons available for bond formation, and good bases are therefore also good nucleophiles. However, in a given set of conditions, the basic properties will take over above a certain threshold strength.
Thus elimination becomes less likely in the series:
-OR
> -OH > RNH2
> NH3 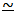 -CN > NO2-
> NO3- > F-
> Cl- > Br-
> I-
-CN > NO2-
> NO3- > F-
> Cl- > Br-
> I-
iii) External conditions: Higher temperatures encourage the formation of smaller molecules and elimination is thus favoured by higher temperatures. (chapter 12).
21.7.5 Unimolecular vs. bimolecular: However, not only do we have to decide between substitution and elimination, we have to decide whether the reaction occurs via a unimolecular or a bimolecular mechanism. Again there are three factors to consider:
i) Attacked
species: Unimolecular mechanisms become less likely in the
series: tertiary > secondary > primary. This can be
predicted because the intermediate carbonium ions on the unimolecular
pathway are decreasingly "stabilised" in this order by positive
inductive effects:
i.e. the ions become decreasingly easy to form in this order because
their formation involves more concentration of charge i.e. more pulling
away of electrons from nuclear control.
ii) Attacking species: Unimolecular mechanisms are more likely to occur with weaker nucleophiles and bases. These give the attacked molecule time to split into ions.
iii) External conditions: Unimolecular mechanisms are favoured by polar conditions/solvents which solvate the intermediate ions i.e. attraction of the ions by solvent molecules encourages their formation.
21.7.6. Nett effect of above factors
Given all the factors which determine the reaction of a haloalkane with nucleophiles/bases, it is hardly surprising that more than one type of reaction is likely to occur in a given set of conditions. It is often very difficult to predict the main course of reaction and therefore difficult to predict the main product - if there is one which majors.
The scheme below summarises the reactions of halogenoalkanes with potassium hydroxide.
Two variables are
considered:
.........i)
branching next to the functional carbon, and
.........ii) type
of solvent (varying from pure water to pure.ethanol
via a
.............continuous
range of mixtures).
The variation of
solvent itself has two effects:
.........first,
water is more polar than ethanol;
.........second,
ethanol favours the formation of an extremely strong base,
.....................the
ethoxide ion CH3CH2O-.
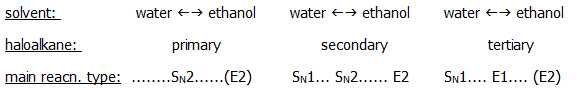
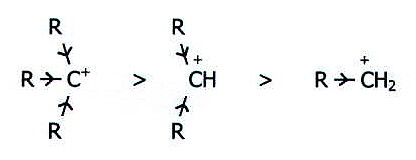
In predicting this pattern, it is useful to have these starting points in you reasoning:
i) Substitution is more likely than elimination, and bimolecular mechanisms are more likely than unimolecular mechanisms.
ii) With primary and tertiary haloalkanes the nature of the haloalkane is a stronger factor than the nature of the solvent. Changes in solvent can be regarded as moving the course of reaction away from the most characteristic type, i.e. away from SN2 for primary haloalkanes, and away from E1 for tertiary haloalkanes.
iii) With secondary haloalkanes the type of solvent is a stronger factor than the nature of the haloalkane. When the solvent is least influential (a mixture of water and ethanol), the reaction is SN2.
21.7.7. Nucleophilic substitution in haloalkanes: examples of nucleophiles.
| Table 21.5. Nucleophiles which react with halogenoalkanes | |||
| Nucleophile | Reagent | Conditions | Main organic product |
| -OH/H2O | Water, or KOH/NaOH in water/ethanol. | Depend on particular haloalkane. Attack by water catalysed by Ag2O | Corresponding alcohol E.g. bromoethane gives ethanol |
| :NH3 | Ammonia in water/ethanol | Again vary. Typical: 100K in sealed tube i.e. pressure |  Corresp. 1° Corresp. 1°  amine 2°
amine 2°  amine 3°
amine 3°  amine 4° ammonium salt.
E.g. C2H5Br
amine 4° ammonium salt.
E.g. C2H5Br  C2H5NH2
C2H5NH2  (C2H5)2NH
(C2H5)2NH  (C2H5)3N
(C2H5)3N  (C2H5)4N+Br-
(C2H5)4N+Br-
|
| amines | see reaction with ammonia above | ||
| -CN | KCN in water/ethanol | reflux | Corresp. nitrile. E.g. bromoethane gives propanonitrile because the carbon of the CN group lengthens the carbon chain |
| -NC | Silver salt | treat with moist salt | Corresp. isonitrile |
| RCOO- | Sodium
salt in water, or Silver salt |
reflux treat with moist salt |
Ester. E.g. Bromoethane gives RCOOC2H5 |
| RO- | Sodium salt in ethanol | reflux | Ether. E.g. Bromoethane gives ROC2H5 |
| NO2- | Silver salt | treat with moist salt | Mixture of corresp. nitrite and nitroalkane |
| NO3- | Silver salt | treat with moist salt | Corresp. alkyl nitrate |
| "R-" | *Grignard reagent | In DRY ether | Alkane. E.g. Bromoethane gives RC2H5 |
Notes:
Note i) *The Grignard reagent mentioned at the bottom of the table is itself formed from a haloalkane. The haloalkane, dissolved in absolutely dry ether, is added to magnesium. It is a vigorous reaction, though often needs gentle warming with the hands to get it going. Alternatively, a crystal of iodine may be needed to start the reaction off.
.................................dry
ether
R-Hal...... +...... Mg......... ........
"RMgHal"
........
"RMgHal"
Once the reaction is going, it must be controlled by adding the haloalkane in only small amounts. Also, a water-cooled reflux condenser must be used to prevent ether fumes from being lost (FIG. 23.3.).
The value of Grignard reagents is that they react as if they had the structure R-MgX+ in ethereal solution (from which they are not usually isolated). Thus they are effectively a source of carbanion nucleophiles, and as such are extremely useful in synthetic reactions (section 23.4.).
21.7.8. More effects of the rest of the molecule
The most dramatic effect on nucleophilic substitution occurs when the halogen atom is attached directly to a benzene ring or a doubly/triply bonded carbon atom. Chlorobenzene, for example, is extremely resistant to nucleophilic substitution. It requires treatment with concentrated sodium hydroxide at 300°C under pressure in order to undergo hydrolysis (i.e. to undergo nucleophilic substitution of the -Cl by -OH).
The reason is that the
high electron density of the benzene ring prevents attack by
nucleophiles on the functional carbon atom. Moreover, delocalisation of
a chlorine lone pair into the benzene ring further increases the
electron density around the carbon. It also gives the C-Cl bond partial
double character making it more difficult to break.
The arguments are similar for halogens attached to doubly bonded and
triply bonded carbon atoms.
In contrast, when the
functional carbon is one removed from the benzene ring or multiple bond
the high electron density of the 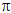 -bonding
system exerts a +I effect about equivalent to that in a secondary
haloakane.
-bonding
system exerts a +I effect about equivalent to that in a secondary
haloakane.
However, the effects are quite different as discussed below in section
21.7.9.
21.7.9. A final comment on relative reactivities
When discussing the relative reactivities of organic halogen compounds, an essential factor is usually completely ignored. The reactivity of a particular organic halogen compound depends on which mechanism of reaction it undergoes (SN1 or SN2). It is therefore also dependent on the other factors which determine the mechanism.
i) In SN1
reactions the rate determining step is the formation of the
carbonium ion. The readiness with which this occurs can be predicted
from information about the activation energy. This in turn can be
gleaned from consideration of the relative stabilities of the
intermediate carbonium ions. Benzenecarbonium ions, and alkenecarbonium
ions are particularly stable because concentration of the positive
charge is reduced by delocalisation of electrons from the p-bonding systems. E.g.

The order of decreasing SN1 reactivity in a
series of haloalkanes (as predicted from the relative stabilities of
the intermediate carbonium ions) is therefore:
C6H5-CH2-Hal > RCH=CH2-CH2-Hal > R3C-Hal > R2CH-Hal > RCH2-Hal
ii) In contrast SN2 reactivity decreases in the order
primary > secondary > tertiary haloalkane.
This is because alkyl groups attached to the functional carbon atom sterically hinder attack by the nucleophile. Benzene rings or mutiple-bonded groups one removed from the functional carbon offer little steric hindrance, though they may reduce the partial positive charge on the functional carbon.
An additional complication is that the order primary > secondary > tertiary is changed when the alkyl groups are themselves branched thus sterically hindering approach by nucleophiles. Thus 1-chloro-2,2-dimethylypropane, a primary haloalkane, is very unreactive.
21.7.10. A final comment on equilibrium
In section 21.7.4.ii., we referred to a relative order of nucleophilic strength. It is tempting to suggest that strong nucleophiles will completely replace leaving groups that would make weak nucleophiles. This is not so. For one thing, good nucleophiles are often good leaving groups (e.g. I-). Moreover, these terms usually refer to rates of reaction.
Thus, in a given nucleophilic substitution reaction, there is another important factor to bear in mind when deciding how good the yield will be: the reaction usually starts far from equilibrium. So, the reaction usually starts with high concentrations of organic reactant and particularly of nucleophile, but zero concentration of products. Even with a good yield of product, the reaction may still be far removed from equilibrium.
Returning to rate of reaction, the conditions are chosen to optimise rate without severely affecting position of equilibrium. Even so, most organic reactions take hours rather than the fractions of seconds generally taken by inorganic reactions.
21.7.11. Other reactions
i) Oxidation: It is possible to make haloalkanes burn. They do so with a slightly smokey flame, but there is no residue. More substituted alkanes like Bromochlorodifluoromethane (BCF) do not burn, and are used in fire extinguishers, though they may give toxic by-products during use.
ii) Reduction: Haloalkanes, and particularly iodoalkanes, may be reduced to the corresponding alkane by the powerful reactant, hydrogen iodide:
R-I....... +....... HI......... ........
R-H....... +....... I2
........
R-H....... +....... I2
iii) With sodium: Alkyl chains may be linked tohether by reaction of haloalkanes with sodium in dry ether:
2RHal....... +....... 2Na......... ........
R-R....... +....... 2NaHal
........
R-R....... +....... 2NaHal
It is likely that this proceeds via carbanions since a 50/50 mixture of R-Hal and R'-Hal gives R-R, R'-R, and R'-R' in a ratio of approximately 1:2:1. The reaction can also link alkyl chains to benzene rings, but again, a mixture of products is obtained.
21.8. QUESTIONS
1) Write balanced equations for examples of all the reactions described in the tables in this chapter.
2) When methane reacts with chlorine, how is the ratio of products affected by changing from excess chlorine to excess methane?
3) If homolytic substitution is a chain reaction, is it necessary, throughout the reaction, to maintain conditions under which free radicals are produced, in order to ensure complete reaction? Explain your reasoning.
4) What products would be present in the mixture produced by cracking butane?
5i) Remembering that energy changes do not provide reasons for the occurrence or non-occurrence of reactions, describe the energy changes which accompany the processes set out in section 21.3.1. (reaction between ethene and bromine).
ii) Using the following
bond enthalpies (in kJmol-1) calculate 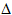 H for
the reaction in i).
H for
the reaction in i).
Br-Br = 193, H2C=CH2 = 598, C-C = 348, C-Br = 276
6) Bearing in mind the states of the reactants, how would you actually carry out the reaction of ethene with bromine to produce 1,2-dibromoethane? How would you use the reaction to distinguish between propene and propane?
7) Which of the electrophiles in table 21.2. will add according to Markowniokoff's rule?
8) Give five reactions of carbonyl compounds (section 22.2) which, when used in conjunction with ozonolysis, may be useful in determining the structure of alkenes.
9) How do you account for the fact that in a compound containing both a double bond and a triple bond, it is possible to add bromine across only the double bond? How might you achieve this selective addition in practice?
10) In view of your answer to question 9, how do you account for the fact that it is possible to stop the addition of halogens or hydrogen halides to alkynes at the stage where one mole of reactant has been added per mole of triple bond?
11) How do you account
for the facts that i) in the absence of free radicals HC CC2H5
gives CH2=CBrC2H5,
but ii) in the presence of free radicals it gives CHBr=CHC2H5?
CC2H5
gives CH2=CBrC2H5,
but ii) in the presence of free radicals it gives CHBr=CHC2H5?
12) How do you account for the fact that 2-pentyne gives trans-2,3-dibromopent-2-ene, and not the cis- product (section 24.3.2.)?
13) Give structures for the two main products of reaction between but-1-yne and hydrogen chloride. How would you control which of these was the main product, and what would be the theoretical basis of this control?
14) Comment on the
following statements: i) Both ethene and benzene contain double bonds
and both therfore undergo similar electrophilic attack.
.............................................................ii)
When an ethene group is attached to a benzene ring, the double bond in
the ethene has more effect on the reactions of the ring than the ring
has on the reactions of the ethene double bond.
15) Draw electron density maps for phenylamine and phenol, using pencil shading to show the differing electron density at various sites in each molecule.
16) Similar amounts of 1-chloro, 1-bromo, and 1-iodobutane are added to a little ethanol solvent in separate test tubes. Dilute aqueous silver nitrate is added to each tube. The tubes are shaken and then placed in a constant temperature water bath. What would you observe in each tube over the course of a few minutes, and how would this illustrate the relative reactivities of the three halobutanes?
16) In sections 21.7.4. and 21.7.5. six factors are described which are involved in the balance between substitution and elimination, and in the balance between unimolecular and bimolecular reaction. For each factor, explain whether rate or position of equilibrium is affected.
17) The reactions of haloalkanes with potassium hydroxide under various conditions are summarised in the scheme in section 21.7.6.. Describe how this pattern is predicted.
18) Ethanol can be made
by treating, say, bromoethane with moist silver oxide. The reactant is
considered to be AgOH, and AgBr is produced. Silver ions are described
as catalysing the nucleophilic substitution reactions of haloalkanes.
Comment. (Section 9.10.)
Unless otherwise stated, all materials in this web version of chapter
21 are © 2007 Adrian Faiers MA (Oxon) MCIPR

What 's the connection between a dozen eggs and
a garden mole?

Answer: Not a lot, really, but see Chapter 1