Recommended by:

Top 20 UK science resources,
The Tutor Website
Recommended by:

Rated:

2010
Listed on the science, engineering and technology section of

'providing you with access to the very best Web resources for education and research, evaluated and selected by a network of subject specialists.'
(Please note that intute closed in July 2011)
Section 1: Essentials (physical chemistry)
CHAPTER 6: THE
CHEMICAL ENERGY TOUR
(an introduction to measures of energy)
NB This chapter has now been
updated to improve browser compatibility.
Please
use the 'send email' link at the top right hand corner of this page to
report any problems.
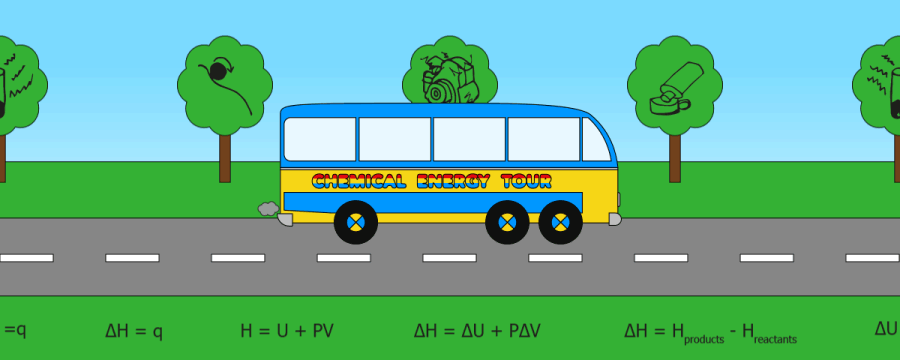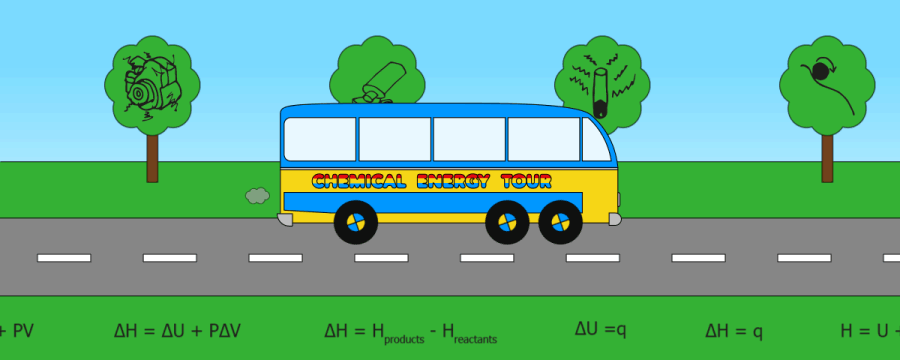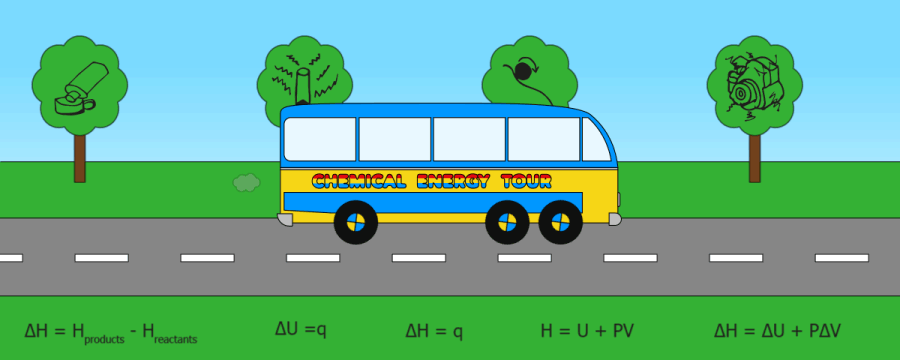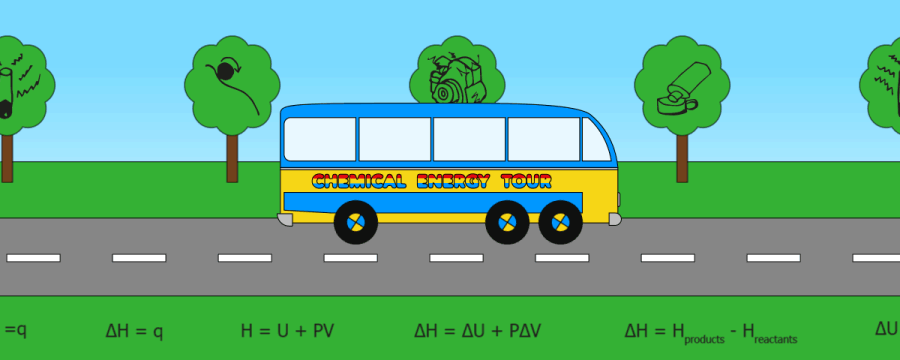
QUICK SKIPS:
(Click on the 'back' arrow to get back to this quick skip section)
Too much energy?
Heat energy(q)
Internal energy
Enthalpy (H)
Enthalpy change (DH)
Positive and negative
enthalpy changes
Standard enthalpy
changes
6.1. THE BALL ON THE
HILL

6.1.1. Energy is one of the most misused and misunderstood concepts in Chemistry. A reaction happens, we are tempted to say, because the products have less energy than the reactants. This is, at best, shorthand. It is like saying that a ball rolls down a hill because it has less potential energy at the bottom.
If we can use the word "because" in the case of the ball, it is in this sense: We can predict that a ball is able to roll down a hill because it has less of what we call potential energy at the bottom, and such behaviour is consistent with previous observations on what we have called potential energy. (See also sections 4.4.5., and 14.4.2.v.).
Perhaps blame for misuse of the word because lies with its ambiguous use in everyday language, and what that ambiguity represents.
However, there is some justification for use of the word "because" in this sense: the concept of energy does cover a large number of apparently different situations; it describes properties in terms of a more general property, and thus unifies observations (sections 2.1.2. and 10.2.1.).
Nevertheless, if we tried to stop the ball from rolling down the hill, we would probably say that we had experienced forces described in terms of gravity rather than experiencing energy (c.f. section 4.1.1.).
6.1.2. Returning to the chemical reaction at the start of section 6.1.1., there is another problem. What do we mean by energy? Energy is defined (somewhat circularly) as the capacity for doing work, but what does that mean in this context?
Just as potential energy is related to the gravitational forces which were involved in the ball rolling down the hill, so chemical energy is related to the electrostatic forces involved in chemical reactions.
The gravitational attraction between the ball and the earth is greater at the bottom of the hill (when the centres of the ball and the earth are closer together) than it was at the top. This corresponds with a decrease in potential energy. Similary, when chemical reactants move to a state of greater electrostatic attraction in the products, a decrease in chemical energy is involved (i.e. change from a higher to a lower energy state).
The opposite is also true. But what is actually measured? There seem to be various measures of energy which could represent chemical energy. The chemical energy tour is waiting to take you away.
6.2. THE COACH SETS OFF: HEAT (q)
6.2.1. The heat energy of a system is a function of the translational, rotational, and vibrational kinetic energies of the atoms and molecules in the system (FIG. 12.1.). Further study would reveal the involvement of electrostatic forces.
In general, the usefulness of any scientific concept is limited by the way we experience or observe it in practice, and by the predictions which it allows us to make. Heat is not only a form of energy which we experience (and can quantify) but along with other forms of energy, it is an invaluable tool for making predictions - even in cases where a study of the forces involved might represent a more detailed description in terms more akin to experience.
In the tour coach you experience forces associated with motion. However, if you were to open the engine compartment, you would feel some of the heat where chemical energy has been inefficently converted into mechanical (kinetic) energy.
Heat
then, is a good
starting point for the chemical energy tour. However, to make heat
applicable to chemical systems, we need to consider other factors, such
as the conditions under which observations and measurements are made.
This results in the definition of another two energy concepts: internal
energy, and enthalpy.
6.3. STOP FOR MORNING COFFEE: INTERNAL ENERGY
6.3.1. The internal energy of a system is the total amount of all types of energy that it possesses, apart from energy arising from its position in space.
Imagine
you have a
vacuum flask completely filled with coffee. If
energy is somehow transferred to the coffee as heat (q), the internal
energy (U) of the coffee will increase. Moreover, in this case of
constant volume, the heat transferred to the system will be equal to
the change in internal energy:
Unfortunately, chemical reactions do not often happen under conditions of constant volume, so internal energy is not a very useful concept for chemists. Another concept, enthalpy, is more useful.
6.4 THE LUNCH TIME STOP: ENTHALPY
6.4.1. The concept of enthalpy (H) has been developed for chemical systems undergoing change, because it most closely relates to the conditions under which most chemical reactions occur. Chemical reactions usually occur at constant pressure not at constant volume. Your tour lunch, for example, may be cooked (a chemical reaction) in a saucepan open to the atmosphere.
In
a laboratory,
reactions are likely to occur in a test tube open to the atmosphere.
Enthalpy has been devised in such a way that, when energy is
transferred as heat to a system at constant pressure, the enthalpy
change is exactly equal to the quantity of heat transferred.
DH = q
This makes enthalpy changes easy to assess (see section 6.4.5.)
6.4.2. Enthalpy (H), at a particular temperature, is roughly defined as the heat content of a system at that temperature (and at a specified pressure).
6.4.3. Enthalpy change (DH) is then the heat that a system would exchange with the surroundings during a change in the system (such as a chemical reaction), if the temperature and pressure of the system were to remain constant.
Simply assessed quantities are not, it would seem, always easy to define.
6.4.4. Mathematical definitions of enthalpy (H) and enthalpy change (DH): We have already said that enthalpy is defined so that, when a transfer of heat energy takes place at constant pressure, the enthalpy change is equal to the quantity of heat transferred.
i.e. 6.4.4a. DH = q
Also, the relationship between enthalpy and internal energy can be expressed in mathematical terms:
i.e. 6.4.4b. H = U + PV
and: 6.4.4c. DH = DU +PDV
where U is internal energy, P is pressure, and V is volume.
What equation 6.4.4c. means in real terms may again be understood by considering transfer of heat energy to a volume of coffee. Unlike that in section 6.3.1., this volume of coffee is in an open vessel, e.g. a pyrex jug, and therefore at constant pressure i.e. atmospheric pressure.
In these circumstances, not only does the internal energy increase (detectable as an increase in temperature) but also the coffee expands slightly. In other words it does work pushing back the atmosphere. The work is equal to the force exerted by the atmosphere x the distance through which it is moved. This in turn is equal to PDV (force per unit area x surface area x distance moved). Remember from your physics that work = force x distance.
6.4.5. Assessing enthalpy changes: The simplicity with which enthalpy change is assessed does justify its clumsy definition. Provided the only work done by a system during a process is work associated with increasing the volume, then at constant pressure the heat absorbed or evolved by the system is equal to the enthalpy change.
In practice, when assessing the enthalpy change during a chemical reaction, the heat of reaction is allowed to alter the temperature of the reacting system. The system is thermally insulated from the surroundings, and the enthalpy change for the reaction is therefore calculated as: the amount of heat that we would need to add to, or remove from, the system in order to return it to its original temperature.
To calculate heat change from temperature change it is, of course, necessary to know the heat capacity of the system. When the reaction takes place in a particular quantity of aqueous solution, the heat capacity is often taken as roughly equal to the heat capacity of the water. (Study question 3, parts i to iii.)
6.5. YOU SAY YES, I SAY NO
(positive
and negative heat changes)
6.5.1. When a reaction takes heat in from the surroundings it is quite naturally described as endothermic. Similarly, a reaction which gives heat out is known as an exothermic reaction. It sounds straightforward, but the introduction of one more simple factor often leads to utter confusion.
Needless to say, the culprit is the following semi-mathematical analysis:
........................................DH = Hproducts - Hreactants
If the products of a reaction have a higher enthalpy content than the reactants, heat will be absorbed during the reaction, i.e. the reaction will be endothermic, and DH will be +ve (a bigger number minus a smaller number).
If the products of a reaction have a lower enthalpy content than the reactants, heat will be given out during the reaction, i.e. the reaction will be exothermic, and DH will be -ve (a smaller number minus a bigger number).
For example, the units of energy are joules or kilojoules (J or kJ). If a reaction absorbs 10kJ of heat from the surroundings it is an endothermic reaction and DH = 10kJ. If a reaction releases 10kJ of heat into the surroundings it is an exothermic reaction and DH = -10kJ. Remember that if we use "heat change" and "enthalpy change" interchangeably, we must be referring to changes which occur at constant temperature and pressure.
6.5.2. A final thought on endothermic and exothermic reactions. Referring back to section 6.2.1., where do endothermic reactions fit into a scheme which claims that reactions happen because the products have less energy? The question is a little unfair at this stage, but nevertheless makes a point. We could continue the energy tour and ask the question again at the end, but it would probably be wiser to take in the sights so far, and resume the tour in a later chapter (Chapter 12).
6.6. STANDARD ENTHALPY CHANGES
6.6.1. Enthalpy changes occur, by definition, at constant temperature and constant pressure. However, to compare the enthalpy change for one process with the enthalpy change for another, we need to set even more strict conditions. For this reason we define standard enthalpy changes.
Standard enthalpy changes are are those which would occur under four standard conditions :
[I]
atmospheric
pressure;
[II] some stated temperature, usually 25°C, i.e. 298K;
[III] the reactants
and products should be in
their standard states, when relevant (i.e. the most stable state at
atmospheric pressure);
[IV] any solutions should be 1 molar.
The
symbol used for
standard enthalpy changes is DHq298.
(C.f. Section 11.8.1)
The quantities of substance involved in the change must also be set. For a change represented by a chemical equation, the chosen amounts are either: the amounts represented in the stoichimetric equation (example 6.6.1.i.); or 1 mole of the substance to which the standard enthalpy change refers by name (example 6.1.1.ii.).
Example i) The standard enthalpy change for the reaction:
...............Ag+(aq)...+...2Cl-(aq)......→......AgCl2(s)
is given per mole of aqueous silver ions reacting and indeed (in this case with perhaps more relevance) per mole of solid silver chloride formed.
Example ii) The standard enthalpy of formation of ethane represented by:
...............2C(graphite)...+...3H2(g)......→....... C2H6(g)
is given per mole of ethane formed.
There are two other standard conditions:
the reactants an the reactants and products should be in their standard states, when relevant (i.e. the most stable state at atmospheric pressure), and solutions should usually be 1 molar.d products should be in their standard states, when relevant (i.e. the most stable state at atmospheric pressure), and solutions should usually be 1 molar.
6.6.2. The application of standard heats (enthalpies) of reaction will be seen in the next chapter, but for now, we shall define a few:
i) The standard enthalpy of combustion for a substance is the enthalpy change when 1 mole of the substance in its standard state undergoes complete combustion under standard conditions i.e. at atmospheric pressure and a defined temperature (usually corrected to 298K).
ii) The standard enthalpy of atomisation for an element is the enthalpy change when one mole of gaseous atoms is formed under standard conditions from the element in its standard state.
iii) Bond (dissociation) enthalpy is the enthalpy change when one mole of a *particular bond in a gaseous compound is broken under standard conditions. It is clearly a measure of covalent bond strength. (*But see 6.6.2.vii below, average bond enthalpy.)
iv) By contrast with bond enthalpy, lattice enthalpy is the enthalpy change when one mole of an ionic lattice is formed from its gaseous ions under standard conditions. It is clearly a measure of ionic bond strength.
Two other particularly important enthalpy changes which need definition (i.e. clarification) are "ionisation energy" and "electron affinity". Definitions are provided below, and in this case it would be a useful excercise to work out precise chemical equations to describe the processes to which they apply.
v)
First ionisation
energy is the energy change when each of the atoms in one
mole of gaseous free atoms of an element loses an electron to form one
mole of gaseous cations (+ve ions).
For magnesium the equation for the process to which first ionisation
energy applies is:
Mg(g) → Mg+(g) + e-
vi) First electron affinity is the energy change when each of the atoms in one mole of gaseous free atoms of an element gains an electron to form one mole of gaseous anions (-ve ions).
You
should also be able
to define successive
ionisation energies. For example, the second ionisation energy
is the energy change when each of the ions in one mole of gaseous,
singly charged positive ions of an element loses an electron to produce
one mole of gaseous, doubly charged positive ions.
For magnesium the equation for the process to which second ionisation
energy applies is:
Mg+(g) → Mg2+(g) + e-
Remember also that you should not use energy and enthalpy interchangeably.
vii) Definitions are not things to learn parrot-fashion. There is not one precise order of words which makes a correct definition. See if you can work out definitions for: standard enthalpy of formation of a compound, standard enthalpy of dissociation of an electrolyte, standard delocalisation enthalpy, standard enthalpy of solvation (of an ion), standard enthalpy of solution, and standard enthalpy of neutralisation (acid/base).
Obviously, the first step is to sort out the precise process for which you are defining the standard enthalpy change. Sometimes this is not quite as easy as it sounds. For bond (dissociation) enthalpies, the process in question is the breaking of a particular type of bond. However, the enthalpy change obviously depends on the environment of the bond.
For example, the energy needed to break an O-H bond in water will be different from that required to break an O-H bond in ethanol. Moreover, the energy needed to break the second O-H bond in water will be greater than that needed to break the first.
We can therefore define two types of bond enthalpy: i) mean (or average) bond enthalpies, and ii) bond dissociation enthalpies for particular bonds in particular environments.
The bond dissociation enthalpies listed in tables are usually mean/average bond enthlapies. They give an indication of the average strength of a bond such as the O-H bond or the C-C bond.
Note that bond enthalpies reflect the fact that it is easier to measure enthalpy changes than it is to measure absolute enthalpies.
6.6.3. Double standards: The process for which we define lattice enthalpy raises another problem. Since bond enthalpies are quoted for bond dissociation, it would be sensible to define lattice enthalpies in terms of the energy change involved when the ions in a crystal lattice are separated to an infinite distance from each other. This is often done, but for some unaccountable and inconsistent reason, chemists also define lattice enthalpy for the reverse process. In fact, this is what A-level examining boards now expect and they mark as wrong values and definitions based on breaking the lattice.
6.6.4. A final important point. It is wrong to use terms like "the energy absorbed" or "the energy released" in your definitions. This information is contained in the positive or negative sign of the numerical value in (k)Jmol-1. What would it mean to absorb or release a negative amount of energy? Your definitions should use the word "change" rather than words like "absorbed", "released", and so on.
6.7. QUESTIONS
1) If a ball is placed on a smooth slope, why does it roll down?
2) What are the differences between heat, internal energy, and enthalpy?
3)
Test tube A contains
5ml of an aqueous solution at 20°C. Test tube B contains 4ml of a
different solution at 20°C. The contents of test tube A are poured into
test tube B and there is a reaction. The final temperature of the
mixture is 20.5°C.
...............i)
If the specific heat capacity of water is 4,200JKg-1K-1,
calculate the overall enthalpy change.
(NB There is a magic formula which enables you to work out quantities
of heat from information like this. However, you are quite likely to
forget it in an exam, and you do not need it anyway. If you understand
specific heat capacity the units are a useful exam aid. Joules per Kg
per K, i.e. Joules of heat needed, per Kg of water being heated, per K
temperature rise of the water.
As a general rule, magic formulae are used as a lazy short cut, when
understanding is simpler than expected, more useful, and longer lasting
- especially when you are under pressure, as in exams.)
...............ii)
In your working, did you show how you calculated the mass of water from
the information given, and the temperature rise in K from the
information given? If not, do so.
...............iii)
What sources of error were there in the experiment described here?
...............iv)
If test tube A had contained hydrochloric acid, and test tube B had
contained aqueous sodium hydroxide, what further information would you
have needed in order to calculate the standard enthalpy of
neutralisation for this particular acid and base? Explain.
...............v)
Was the neutralisation endothermic or exothermic and explain how you
deduced this from the above information?
...............vi)
In this reaction, which had the greater enthalpy content, the reactants
or the products?
...............vii)
From you answer to part vi show whether the numerical value of the
enthalpy change was positive or negative.
4) Write down the definitions you worked out in section 6.6.2.v.
5) Work out and write down a definition for standard enthalpy of reaction.
6a) Write down the chemical equations you worked out in section 6.6.2. iii and iv.
b) It would have been ambiguous to leave out the words "gaseous" and "free" from the definitions (6.6.2.iii. and iv.). Explain why, by describing what extra energy terms would be included in the first ionisation energy of sodium and in the first electron affinity of chlorine, if the two words were omitted.
7) Work out and write down definitions for second ionisation energy and second electron affinity.
Unless
otherwise stated, all materials in this web version of chapter 6 are ©
2007 Adrian Faiers MA (Oxon) MCIPR
Animations © 2016 Samuel Faiers

What 's the connection between a dozen eggs and
a garden mole?

Answer: Not a lot, really, but see Chapter 1