Recommended by:
Top
20 UK science resources,
The Tutor Website
Recommended by:

Rated:

2010
Listed on the science,
engineering
and technology section of 
'providing you with access
to the very best Web resources for education and research, evaluated
and selected by a network of subject specialists.'
(Please note that intute closed in July 2011)
Section 1: Essentials (physical chemistry)
CHAPTER 5: STATES
OF MATTER
NB This chapter has now been
updated to improve browser compatibility.
Please
use the 'send email' link at the top right hand corner of this page to
report any problems.
QUICK SKIPS:
(Click on the 'back' arrow to get back to this quick skip section)
Solids
X-ray diffraction
Giant molecular
structures
Ionic, metallic and
simple molecular lattices
Liquids
Gases
Vapour Pressure
Avogadro's, Boyle's and
Charles's Laws and ideal gas equation
Kinetic Theory of gases
Real gases
Critical temperature
and pressure and liquefaction of gases
Revision
worksheet 2: Bonding
Answer sheet 2:
Bonding (follow link on home page)
5.1. WHAT WE SEE
5.1.1. We do not see individual atoms or ions, we do not see bonds, and we rarely see idividual molecules. We do see collections of atoms, of ions, and of molecules. Usually they are solids, sometimes they are liquids, and on rare occasions we see gases. Whether we see them or not, it is the collective properties of atoms, ions, and molecules which we experience.
But what is the arrangement of the atoms, ions, or molecules in solids, liquids and gases? If we knew, it might increase our understanding of the collective properties we experience and observe.
5.1.2. The main difference between solids, liquids, and gases is somewhat predictable. In solids the constituent particles tend to be close together and relatively unable to move. In liquids they are further apart and moving quite freely. In gases they are even further apart and buzzing about all over the place (not a technical term).
5.2. SOLIDS
5.2.1. Solids are a good place to start because we experience them most overwhelmingly. To examine the arrangement of the constituent particles in solids we do, however, need some fairly powerful methods.
These include X-ray diffraction, to a lesser extent electron diffraction and sometimes other methods such as NMR (nuclear magnetic resonance) and ESR (electron spin resonance) can give useful information.
5.2.2. X-ray diffraction uses X-rays, which have a similar wavelength to the size of atoms, ions etc. When the X-rays travel past atoms, ions and so on, they are diffracted, or bent, just as visible light rays are diffracted when they pass the edge of a solid object. X-rays hitting an ionic crystal can, by way of example, be used to find the arrangement of ions in the crystal.
The X-rays start off with their waves in phase (see FIG. 5.1). However, rays diffracted by individual ions in a crystal may or may not be in phase with rays diffracted by other ions. When they are, they reinforce each other. When they are not, they tend to cancel each other out. The condition for reinforcement is shown in FIG. 5.1.
The nett result is that particular arrangements of ions produce particular patterns of reinforced X-rays. The X-ray patterns can be detected on, for example, photographic plates. Understanding the patterns allows the arrangement of ions in the crystal to be deduced.
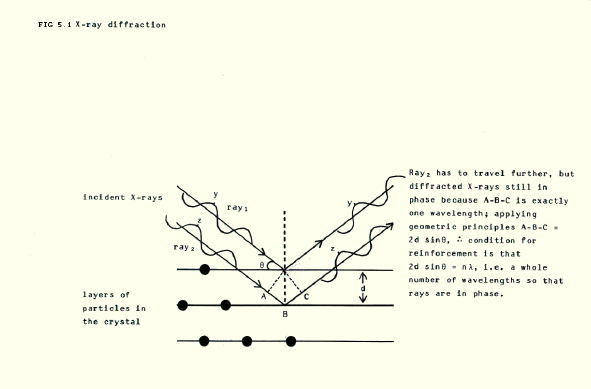
The same technique can be used to find the arrangement of ions in metals and of atoms in molecular crystals.
5.2.3. Giant molecular structures: Elements such as carbon and silicon may exist as solid giant molecular structures, as may compounds such as silicon dioxide, proteins and plastics, to name but a few. There are many different arrangements of atoms in such structures and it is difficult to talk about common patterns. The best we can do is look at an example.
i) Carbon is a frequently discussed example. The two main structures are diamond and graphite. It is hard to believe that one element could exist in two such vastly different forms. The differences are reflections of the different bonding and different arrangements of atoms in the two structures.
In diamond the atoms are sp3 hybridised. Each carbon is bonded tetrahedrally to four other carbon atoms by strong covalent bonds. (FIG. 5.2.)
In graphite the atoms are sp2 hybridised. Each carbon is bonded triagonally to three other carbon atoms by strong covalent bonds with multiple character. (Fig. 5.2.)
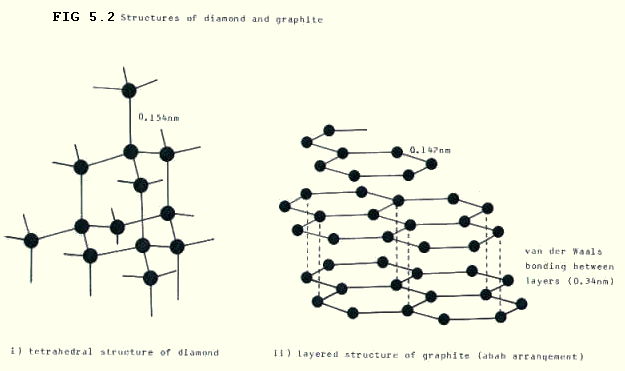
The properties do overlap. Obviously the strong covalent bonds are difficult to break in both structures and both have extremely high melting points (and boiling points). The strength of the bonds also explains why diamond is one of the hardest substances there is. Moreover, it is difficult to distort the bonds at all, without breaking them: diamond is brittle as well as hard.
But graphite is soft. This is a little surprising at first sight. The covalent bonds are slightly harder to break than those in diamond, because they have multiple character. However, the carbon atoms are arranged in covalently bonded layers (FIG. 5.2.) and the bonds between one layer and another are weak Van Der Waals bonds. It is these weak bonds which are broken first.
Though it is not quite this simple. Graphite is soft only when it contains air molecules as an impurity. The air molecules occur between the layers and act as "rollers" allowing the layers to easily slide over each other and making graphite soft and slippery. It is even used as a lubricant. Imagine using diamond dust as a lubricant; it is actually used as an abrasive.
Note that in high vacuums, the air molecules are removed from graphite, causing the layers to stick.
The rather unusual bonding in graphite accounts for another property which it certainly does not share with diamond. The bonding electrons in diamond are strongly confined to the covalent bonds. Diamond does not conduct electricity.
However, the p-orbital electrons in graphite are mobile in an electric field. Thus graphite does conduct electricity. The same p-electron cloud reflects light and accounts for graphite's metallic lustre. The p-bonding orbital is very similar to a metallic bonding orbital in these respects.
Since 2002 it has become possible to create single layers of graphite (1 atom thick). The material, known as graphene, has a range of useful properties. It is, for example over 200x stronger than steel by weight and it has useful electrical/electronic properties, so much so that worldwide trade in graphene was estimated at £9bn in 2014 with most of the applications being in electronics and composites.
5.2.4. Ionic, metallic and simple molecular lattices: This may seem like a diverse bunch of structures to consider in the same breath. We already know from the previous chapter that we are dealing with a wide variety of bond types. It is just because we are clear about these distinctions that we can risk this lumping together.
The point is this. Whether we are dealing with oppositely charged ions attracted to each other, or with positive ions attracted to a single bonding cloud, or even with simple molecules attracted by relatively weak van der Waals forces, the packing particles will try to get close to each other. There are surprisingly few ways of achieving this. As a result, particles of whatever type pack together in a small number of commonly occurring arrangements.
Unlike giant molecules, ionic, metallic and simple molecular lattices show a few repeating patterns. There are fourteen arrangements in metals, of which three are shown below.
i) Body-centred cubic arrangements are found in caesium chloride amongst ionic compounds and in iron, manganese and the alkali metals amongst metals.
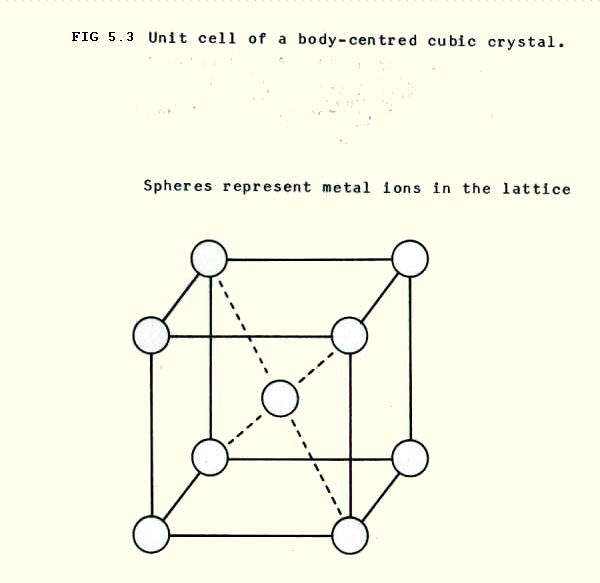
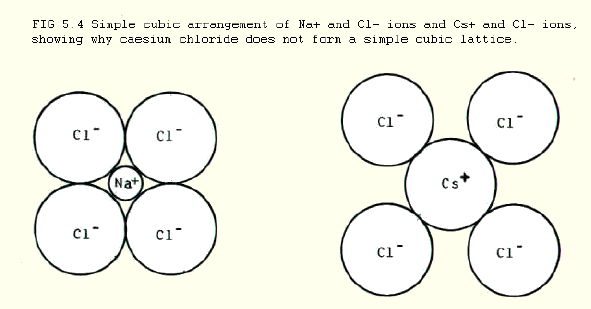
In the case of ionic compounds like caesium chloride, the single anion shown in FIG. 5.3. can be pictured as part of a simple cubic lattice of anions within a simple cubic lattice of metal ions. The structure is said to show 8:8 co-ordination: i.e. each anion is surrounded by 8 cations, and each cation is surrounded by 8 anions.
In contrast with caesium chloride, sodium chloride forms a simple cubic structure. (FIG.4.2.): a simple cubic arrangement in caesium chloride would not be as tightly packed because the caesium ion is bigger than the sodium ion.
Metals which pack according to the body centred cubic arrangement use 68% of the space. This is not one of the, so-called, close-packed structures, but it is still a fairly efficient use of space. The co-ordination number is 8.
ii) Hexagonal close-packing is found in nickel, zinc and magnesium amongst other metals. It uses 74% of the space, and the co-ordination number is 12.
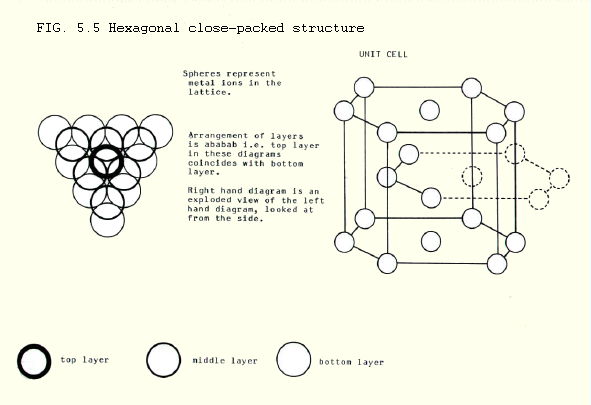
iii) Cubic close-packing is found in aluminium, copper, lead and silver amongst the metals. It is also the arrangement taken up by iodine molecules in solid iodine. Like hexagonal close-packing, it uses 74% of the space, and the co-ordination number is 12.
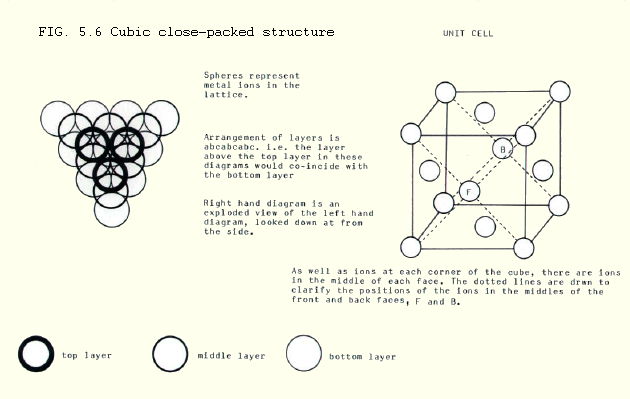
Owing to the nature of the unit cell in this arrangement, cubic close-packed structures are also known as face-centred cubic structures. One example of a face-centred cubic lattice is iodine in which covalent I2 moleclues are held in a lattice by weak intermolecular forces, as described in Chapter 4.
Note from FIGS. 5.5. and 5.6. how the units are packed just as tightly in the two cases. The underlying difference is the pattern of repeating layers: abab or abcabc. These produce different unit cells.
iv) In ionic substances the patterns are complicated by the fact that more than one type of ion is present in the structure, and even in the case of binary compounds these may not be present in a ratio of 1:1. The result is that ions in ionic crystals may often be seen as a particular arrangement of one ion within a particular arrangement of the other.
We have already seen that the body centred cubic structure of caesium chloride can be seen as a simple cubic arrangement of caesium ions within a simple cubic arrangement of chloride ions (or vice versa). Another example is calcium fluoride. This may be seen as a face centred cubic arrangement of calcium ions within a simple cubic arrangement of fluoride ions.
Even the apparently simple cubic structure of sodium chloride can be seen in a different way. It can be seen as a face-centred cubic arrangement of sodium ions within a face-centred cubic arrangement of chloride ions.
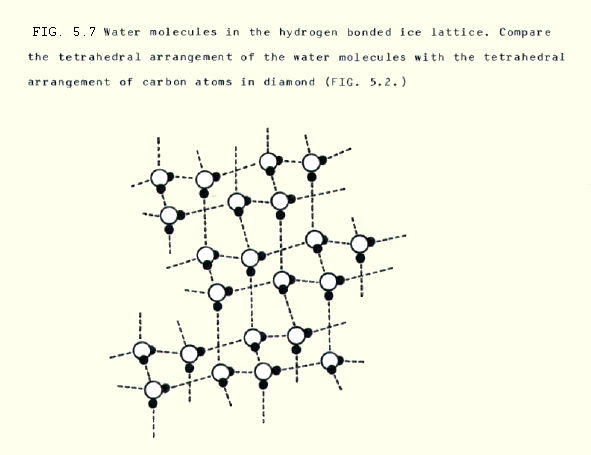
Only one clear example of a primitive cubic structure is known: polonium metal. It should also be said that lattices of simple molecules show even more variety than ionic compounds. For example, the structure of ice is very much determined by the shape of the water molecules and the directional nature of the hydrogen bonds between them.
5.2.5. Despite the similar arrangements of particles in ionic, metallic and simple molecular lattices, the properties are quite different. This is a function of the different bonding (table 4.1.).
i) Ionic crystals are hard and brittle because the bonding is strong and is difficult to distort without breaking. The strong bonding also leads to the high melting and boiling points.
There are no mobile ions or electrons in ionic lattices so the compounds are not electrical conductors in the solid state. However, when molten, the ions are mobile and able to transport charge. Molten ionic compounds are therefore good electrical conductors.
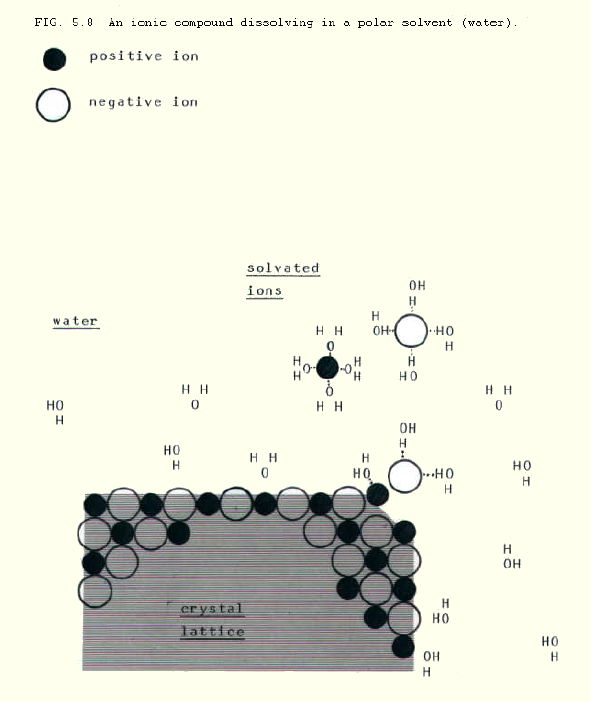
Ionic compounds are soluble in polar solvents because several polar solvent molecules (eg water) will pull each ion away from the lattice. It is the combined strength of attraction of several solvent molecules for each ion that may overcome the strong ionic bonding. Since such solutions contain mobile ions, they conduct electricity.
However, the attraction of non-polar solvent molecules (eg tetrachlormethane) for ions is so weak, that even large numbers of them cannot pull ions away from the lattice. Ionic compounds therefore tend to be insoluble in non-polar solvents.
ii) Metallic substances tend to be malleable and ductile, that is they can be hammered into different shapes, or stretched into shapes such as wires. This is because there are no rigid, directed bonds and groups of ions can be forced into new positions within the cloud of bonding electrons.
Nevertheless, the bonding must not be thought of as weak overall. Metals can have high melting points and boiling points, although the "best" metals in chemical terms (highly electropositive elements) have quite low melting points and boiling points. In fact, electropositive metals do not exemplify many of the characteristics we associate with everyday metals like steel.
Not only are ions within the electron cloud moveable, but also the electrons in the orbital are mobile in an electric field. This makes metals good electrical conductors. The mobility of the bonding electrons also makes metals good conductors of heat. Where the metal is hot, vibrating ions excite electrons. The motion is then transmitted via the bonding orbital electrons to ions in a cooler part of the lattice.
iii) Simple molecular crystals of substances such as iodine, napthalene and ice tend to be soft because the weak bonds between one molecule and another are fairly easily broken. In iodine these bonds are van der Waals bonds, whereas in ice they are hydrogen bonds.
Moreover, the substances have low melting points and boiling points again because the weak bonds between one molecule and another break easily.
In no state do straightforward molecular substances contain mobile electron or ions, thus they do not conduct electricity.
They do not dissolve in polar solvents because in this case the solvent molecules are too strongly attracted to each other for them to attract non-polar molecules away from the lattice. However, in non-polar solvents both the solute-solute attractions, and the solvent-solvent attractions are weak. Simple molecular solids may therefore dissolve in non-polar solvents when enough sufficiently strong bonds form between solvent and solute molecules for the solute molecules to be attracted away from the lattice.
The balance is fine, and another factor is often important. When a substance dissolves there may be an increase in freedom and a dissipation of energy within the system. Observations show that naturally occurring processes always involve an increase in overall dissipation of energy (chapter 12).
Such changes in the dissipation of energy are even important in finely balanced cases of ionic substances dissolving in polar solvents. Sometimes ionic substances dissolve in water even when the total attraction of water molecules for ions in the lattice is slightly weaker than the attraction of the ions for each other. They do so when there is an overall increase in the dissipation of energy.
5.3 LIQUIDS
5.3.1. The brief comparison of solids, liquids and gases in section 5.1.2. was misleading. The comparison may be useful for solids, liquids and gases well away from their melting points and boiling points. However, it would lead to wrong expectations about the microscopic changes which take place at these transition temperatures.
Since melting (or solidification) and boiling (or condensation) are the processes during which the key changes from one state to another take place, we must understand the microscopic changes which occur during the processes. Otherwise, we shall not understand the key differences between solids, liquids and gases.
5.3.2. The brief comparison may lead us to expect that the basic particles in a solid become much further apart on melting. This is not correct. They may become slightly further apart, but not much. When ice melts, the water molecules actually become slightly closer together. Water reaches its maximum density around 4°C.
Considering the ice to water process the other way round, when water cools the hydrogen bonds which create the ice lattice (see Fig 5.7 above) force the molecules apart slightly. This causes ice to have a lower density and explains why ice floats.
We may also have a wrong idea about the increased motion of the particles when a substance melts. Most commonly it is an increase in temperature which causes a substance to melt. As the temperature is increased the particles vibrate more and more vigorously. We might think that the particles vibrate far more vigorously when it melts. They do not.
The key observable difference is in freedom. In solids the particles vibrate about a fixed point in the lattice. They are bonded to adjacent particles in the lattice. As the temperature increases the particles vibrate more and more vigorously. This much is true. But they do not suddenly vibrate far more vigorously when a substance melts. What happens is that the vibration reaches a critical point which allows the particles to break free of their fixed positions in the lattice. In other words, bonds are broken.
Nevertheless, this does not mean that each particle instantaneously does a few lengths of the pool. It merely means that each particle is free to move anywhere within the liquid. Near the melting temperature it would probably take the particles a long time to move far away from where they started, excluding the effects of any external forces such as gravity.
However, "long time" and "far away" are relative terms, bearing in mind the frequency of vibrations (around 1013 Hz) and the sizes of atoms and molecules. Each time the particles move, they move by only a fraction of their radius, but since they do this around 1013 times a second, their average position can change fairly rapidly on the type of time scale we are used to.
The key fact remains that liquids do not contain permanent bonds between one particle and another. (Note we are not talking about bonds within molecules of a particular liquid. These remain permanent.) This means that liquids are very subject to external forces. Under the influence of gravity, or other forces, they adopt the shape of their container and flow easily. However, since their particles are already close together, they are not easily compressed.
Another point should be made about liquids. Although they do not contain permanent bonds between one particle and another, at any given instant there may be a considerable amount of structure with many particles bonded to others. The surface of a liquid is particularly likely to be structured.
In water at room temperature, for instance, there can be fairly extensive tracts of molecules hydrogen bonded in ice-type lattices.
In solutions, solute-solvent bonds can produce a fair degree of structure. To a large extent a hydrated ion behaves as single liquid particle. It also structures surrounding water molecules beyond its immediate hydration shell.
However, as the temperature of a liquid reaches its boiling point, the amount of structure decreases, so lack of structure should not be taken as a fundamental difference of gases. For such differences we should look at the events which accompany the change of state between liquid and gas. Events which thus define basic properties of liquids as well as those of gases.
5.4. GASES
5.4.1. The bulk differences between liquids and gases are well known to us and, this time, the brief comparison of solids, liquids and gases (section 5.1.2.) is more useful.
The main change we would expect when a liquid boils is that the particles suddenly become a lot further apart and suddenly start moving a lot more freely. This does happen.
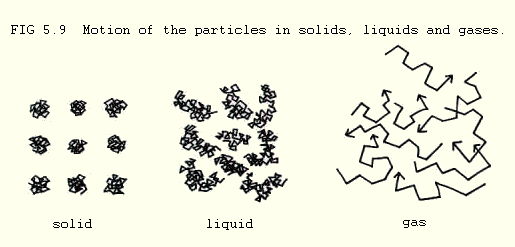
In a liquid, there are always a few particles which are moving around with enough vigour to escape the surface of the liquid and enter the atmosphere. When they do so, they immediately have as much space and as much freedom to move as all the other gas particles, though they may return to the liquid.
As the temperature of the liquid is increased, the proportion of particles in the liquid which are capable of escaping increases and the liklihood of a once vapourised particle returning to the liquid decreases. At the boiling point, all the particles are capable of escaping and the chances of one returning are minimal.
5.4.2. Vapour pressure is a concept used to link these events with our experience of the bulk properties. The pressure at the liquid surface caused by particles leaving the liquid is called vapour pressure. Since the proportion of particles able to escape the liquid increases with temperature, so, too, does vapour pressure. It comes as no surprise that the vapour pressure reaches atmospheric pressure at the boiling point.
Once gaseous, the particles move rapidly and randomly through large amounts of space, changing direction only when they collide with other gas particles or with surfaces of one kind or another. When the surfaces are those of a container, it is bombardment by gas particles which is experienced as the gas's pressure. Gases are easily compressed because of all the space between one particle and another (appendix iii).
5.4.3. We have implied that substances immediately acquire all the characteristic properties of gases once a liquid boils. This is not the case. We can clarify the characteristics of gases which are not found in liquids by observing the changes which occur on boiling. However, a gas does not have these characteristics in full measure near the boiling point of the substance.
In order to develop some of the theory used to describe the behaviour of gases, the characteristics of gases are idealised to such a degree that they never possess them in absolutely full measure. The characteristics attributed to "ideal" or "perfect" gases are, then:
i) Gases consist of particles in rapid random motion.
ii) The gas particles have negligible volume compared with the space they occupy.
iii) Collisions of gas particles with each other and with the walls of the container are perfectly elastic.
iv) Inbetween collisions the particles experience no forces from each other or from the walls of the container.
v) The speed of the particles is proportional to their temperature.
These assumptions apply to all ideal gases, so it follows that the average kinetic energy of any gas particles at a given temperature will always be the same, whatever the gas.
These characteristics attributed to ideal gases at a microscopic level are also paralleled by ideal behaviour at a bulk level.
5.4.4. Bulk properties of ideal gases: The following properties have been described for ideal gases by three historical scientists, Boyle, Charles, and Avogadro:
i) Avogadro's law summarises observations that equal volumes of any gas, at the same temperature and pressure, contain the same number of moles of gas particles.
i.e.
V  n
n
It is also true that 1 mole of any gas occupies the same volume at a given temperature and pressure. At atmospheric presure and 273K, the volume is 22.4dm3.
ii) Boyle's law summarises observations that the volume of a fixed mass of gas is inversely proportional to the pressure, provided the temperature remains constant.
i.e.
V  1/p
1/p
or pV = a constant.
iii) Charles's law summarises observations that the volume of a fixed mass of gas is directly proportional to its absolute temperature, provided the pressure remains constant.
i.e. V  T.
T.
It is by plotting volumes of gases against temperature, that the absolute temperature scale is defined. If volume is plotted against temperature in °C and the plot extrapolated back, the graph predicts that gases have zero volume at approximately -273°C. This suggests a natural absolute zero of temperature, and this is the basis of the Kelvin scale.
iv) The ideal gas equation combines the above three observations in one summarising statement:
pV  nT
nT
........................................or:
pV = nRT
where R is a constant known as the universal gas constant. Its value is 8.31JK-1mol-1.
5.4.5. Ideal gas behaviour "under the microscope": A clever application of the ideal gas characteristics described in section 5.4.3. leads to an interesting equation:
.......................................................pV = 1/3nMc2
where M is the molar mass and c2 is the mean square speed of the particles, i.e. the average value of the squares of the speeds.
5.4.6. Kinetic theory of gases. This equation is the master stroke of the kinetic theory of gases. The characteristics set out in section 5.4.3 are the underlying assumptions of the theory, and it is largely consistent with observations of the bulk properties of ideal gases:
i) Avogadro's law. Compare equal volumes of different gases at the same temperature and pressure:
...................................................p1V1 = p2V2
........................................Thus:
..........................................1/3n1M1c12 = 1/3n2M2c22
However, Mc2 is the same for both gases, since the average kinetic energies (½Mc2) are the same for both gases.
.................Thus:.........................2/3n1 = 2/3n2
............. .......so:.............................n1 = n2
i.e. kinetic theory is consistent with the observations summarised by Avogadro's law.
ii) Boyle's law. Since n and M are constant for a given mass of gas, and since c2 will remain constant at constant temperature, pV will be constant according to the kinetic theory equation.
iii) Charles's
law. Since n and M are constant for a given mass of gas, at
constant pressure, V  c2. Since c2 is
proportional to the temperature, kinetic theory is compatible with
Charles's law.
c2. Since c2 is
proportional to the temperature, kinetic theory is compatible with
Charles's law.
iv) More interestingly the kinetic theory equation can be combined with the ideal gas equation:
...............................................1/3nMc2 = nRT
.................................giving:
.......................................................c2 = 3RT/M.
In other words, the r.m.s. speed of gas particles is proportional to the square root of the temperature and inversely proportional to the square root of the molar mass.
5.4.7. Distribution of speeds: We have talked about mean speeds of particles in a gas, but the particles in a gas have a range of speeds. There are more particles with the r.m.s. speed than any other speed as shown in the graph below.
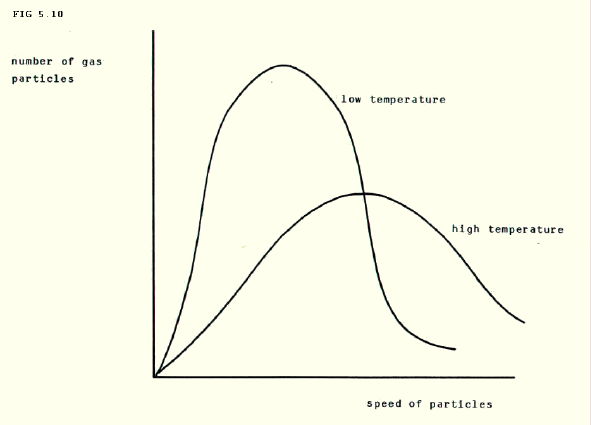
The graph also shows the effects of increasing temperature. At the higher temperature the graph is flatter, showing that there is a greater range of speeds. Secondly, the peak is moved to right, showing that the r.m.s. speed is higher.
5.4.8. There are several experiments which can be used to demonstrate the distribution of speeds in a gas. One, the Zartmann experiment uses the apparatus shown in the diagram below.
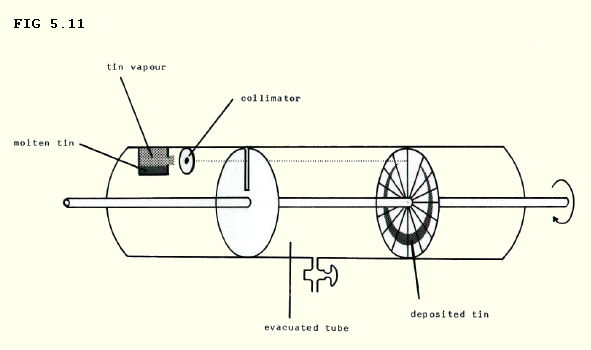
The section of the numbered wheel hit by a particular tin atom depends on the atom's velocity and the speed of rotation of the wheel. If the speed of the wheel is chosen carefully, the distribution of speeds in the gas is reflected by the pattern of tin deposited on the numbered wheel.
5.4.9. Energy: The Maxwell-Boltzmann distribution of speeds in FIG. 5.10. can easily be changed into a distribution of kinetic energies since k.e. = ½mv2, and applied to this situation, this gives k.e. = ½Mc2, as we have already seen above.
Since much discussion about gases involves discussion about the effects of heating and cooling, energy is a useful concept when trying to understand the behaviour of gases.
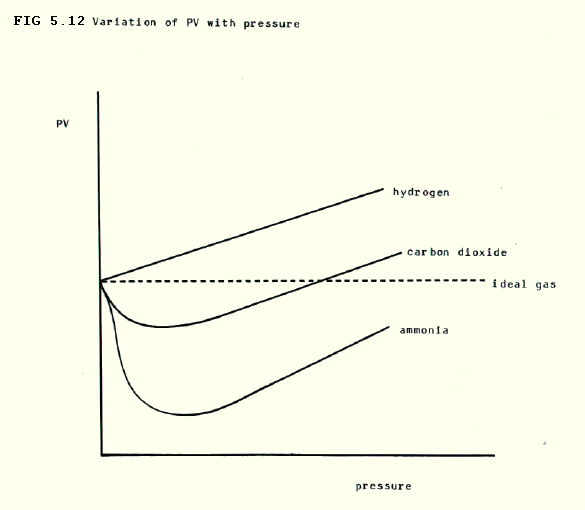
5.4.10. Real gases: Throughout our discussion of ideal gases, we have implied that ideal behaviour is rare. It is most likely to occur at low pressure and high temperatures when the gas particles are furthest apart and moving most rapidly. Applying the kinetic theory equation to 1 mole of gas, pV/RT should obviously equal 1. However, as the pressure on a real gas is increased the ratio tends to change as shown in FIG 5.12.
The main deviations from the ideal behaviour described by kinetic theory are:
i) Gas particles do not occupy negligible volume, especially at high pressures and/or low temperatures when the particles are closer together/moving less rapidly.
ii) Gas particles do exert forces on each other. Again this is especially so at high pressures and/or low temperatures.
FIG. 5.12 suggests that, as the pressure is increased, initially the reduction of pressure by attractive forces is important. However, as the pressure gets even higher, the volume of the particles becomes more important. A modification of the ideal gas equation by van der Waals tries to take these two deviations into account:
For one mole of gas:....(P + a/V2)(V - b) = RT
The constant, a, takes into account attractive forces between particles and constant, b, takes into account the volume occupied by the particles. A quantity is added to the actual pressure to compensate for its lower value, and a quantity is subtracted from the actual volume to compensate for the fact that it includes the volume of the gas particles.
5.4.11. Liquefaction of gases: Liquefaction is the clearest example of non-ideal behaviour in gases resulting from attraction between particles! Below a so-called critical temperature gases can be liquefied by pressure alone. The pressure needed to liquefy a gas at the critical temperature is called the critical pressure.
Plots of pressure against volume at various temperatures were first made by a scientist called Andrews. They represent graphically some of the changes during liquefaction graphically.
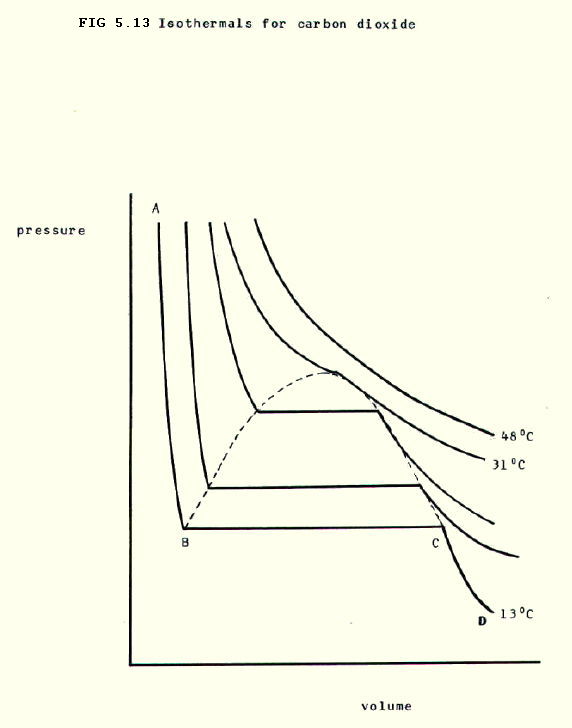
The Andrews curves for Carbon dioxide show remarkably ideal behaviour at 48°C. At 13°C, however, the behaviour is reasonably ideal along AB, but along BC there is a very large change in volume for a very small change in pressure. This represents liquefaction. Section CD shows that it is difficult to compress liquids.
Judging from the shapes of the graphs at other temperatures, it becomes increasingly difficult to liquefy carbon dioxide at temperatures above 13°C and the critical temperature appears to be around 31°C.
5.5. A FINAL POINT
By this stage you should be able to picture solids, liquids, and gases at a microscopic level. This is very important. To understand chemical processes and reactions, you should always try and picture events at this level. In other words you should try and construct a model in your mind.
We have already said that models can rarely be called wrong if they help you to make correct predictions. For this reason it is better to have a somewhat questionable picture of what happens at a microscopic level than no picture at all. Go on using a model until it fails to fit the facts. Then is the time to modify it, and then your modified model may help you to make even more accurate predictions.
5.6. QUESTIONS
1) In graphite the reflection of light by the p-orbital bonding electrons gives it a metallic lustre. How would you relate diamond's optical properties (transmission AND reflection) to its structure?
2) The arrangement of atoms in giant molecules shows more variety than there are differences between the arrangement of ions in metallic lattices and ionic lattices, yet giant molecules contain only covalent bonds whereas metallic and ionic bonds are totally different. Discuss this statement ignoring those giant molecules which contain other types of bond such as hydrogen bonds, van der Waals bonds etc.
In your answers mention as many examples as you can, but do not draw complex diagrams.
3) Write definitions of a) melting and b) dissolving and add any comments necessary to distinguish between the two processes.
4) Many physical chemistry books start with the kinetic theory of gases. This is because chemistry is a science and therefore based on experiment: kinetic theory provides an experimental approach to the nature of atoms and molecules. With hindsight, what might you have deduced about atoms, molecules, moles etc. from observations on gases?
5) Describe melting and boiling with reference to energy, taking care how you use the word "because" (section 6.1.1.).
6) In section 5.4.3. we deduced from the basic assumptions of kinetic theory that the average kinetic energy of gas particles at a given temperature will always be the same, whatever the gas. How does this follow?
7) What measurements could be made, and how could they be used, to obtain a quantitative graphical representation of the results from a Zartmann experiment? (Section 5.4.8.)
8) In similar circumstances which will diffuse through a pinhole most quickly, ethane, oxygen, or nitrogen? Explain. Which will diffuse most slowly?
9) When gas is released suddenly from a region of high pressure to a region of low pressure there is a cooling effect. If gases behaved as ideal gases this would not happen. Explain why the cooling occurs.
10) Explain in more detail the modifications by van der Waals to the ideal gas equation.
Unless otherwise stated, all materials in this web version of chapter 5 are © 2007 Adrian Faiers MA (Oxon) MCIPR

What 's the connection between a dozen eggs and
a garden mole?

Answer: Not a lot, really, but see Chapter 1