Recommended by:

Top 20 UK science resources,
The Tutor Website
Recommended by:

Rated:

2010
Listed on the science, engineering and technology section of

'providing you with access to the very best Web resources for education and research, evaluated and selected by a network of subject specialists.'
(Please note that intute closed in July 2011)
Section 4: More Everyday Chemistry
CHAPTER 28: SCIENCE
FICTION
(correct and incorrect application of chemical theory
to everyday issues)
NB This chapter has now been
updated to improve browser compatibility.
Please
use the 'send email' link at the top right hand corner of this page to
report any problems.
Also, please note this chapter still
needs updating though broken links have been removed, 13 Feb 2018
QUICK SKIPS:
(Click on the 'back' arrow to get back to this quick skip section)
Pollution in the
chemical industry
Pollution other
causes, including farming, road traffic and plastics
Food additives
Organic farming
Homeopathic
medicines
Alcohol
Bio-fuels
A conclusion
28.1. INTRODUCTION
In one way or another, chemistry affects us all. Sometimes it affects us positively, sometimes negatively. It affects us positively when we use one of the chemical industry's products to our advantage, or when we use our own grasp of chemistry to avoid harm to ourselves by a potentially dangerous chemical. It affects us negatively when we are subjected to the chemical industry's pollution, or when we refuse to accept a fact because it does not fit in with our own particular understanding of chemical theory.
Clearly, the balance between the positive and negative effects is nothing to do with chemistry itself, except that chemistry is a particular way of describing and understanding things that has been devised by people. It follows that the balance between positive and negative effects is controlled by people. Sometimes, it is in our own individual control.
In addition, the application of scientific theory involves value judgements. Scientists, including chemists, often believe that there is a line between scientific arguments and value judgements. If there is a line, it is simply a line beyond which the variables appear too infinite for simple application of scientific method.
However, this does not mean that it is a line beyond which scientific method has no place. Indeed, it is a line which every honest and responsible scientist must frequently cross. It is a line beyond which emotions, ethics, and a host of other complex data must be included in the scientist's reasoning, alongside "simple" scientific data.
When the line is crossed, it must be made clear how each piece of information has been used in the argument. Frequently, the precise roles of various factors in an argument are fudged by flaky (or devious) use of scientific language, or everyday language, or both.
28.2. CHEMISTRY AND THE
ENVIRONMENT
28.2.1. Pollution is one of the first words to come to mind when mention is made of chemistry and the environment. The picture is of a faceless industrial organisation pumping some kind of noxious substance into the environment at the expense of the innocent individual. Sometimes it is a valid picture. Sometimes the innocent individual shares at least part of the responsibility.
28.2.2. If an oil rig pumps water polluted with oil into the sea the responsibility seems clearly to lie with the oil company. They should clean up the water before discharging it into the sea, and it might be tempting to think that the oil company is cutting costs in order to maximise profits. Even if accidental, cost cutting might be a contributory factor.
However, the company might argue that it is cutting costs in order to satisfy demand for low prices from customers like us. Moreover, in a leaflet on the environment issued by one major oil company in the late 1980s (showing, at least, that environmental concerns are not as recent as some might think), the following statement appeared: "The environmental cost of industry is part of the total cost of satisfying human wants by producing goods and services". Is this fair? Is pollution an inevitable consequence of our demands on industry, or is this particular company dumping responsibility in the same way as it might dump waste?
The same leaflet went on to describe considerable measures taken by the company to avoid harming the environment, including:
i) Prevention through training, good equipment and design, and appropriate operating procedures. For example, during drilling, well pressures are contained by the weight of drilling mud used. If the well pressure overcomes the mud weight, large mechanical devices known as blow-out preventers are actuated, and these seal the well.
Since blow-out would cause loss of valuable oil and its containment would be needed before more oil could be usefully extracted, there was strong financial pressure on the company to take preventative measures. It is always easier for companies to adopt a moral position when there is financial pressure to do so.
ii) Instant call-up of a team of as many as 50 staff members, plus a contracted labour force up to 1,000, to deal with major incidents such as oil spills.
iii) A worldwide research programme into the use of dispersant chemicals to treat spilled oil, and into their effect on marine life.
iv) Daily checking and strict quality control of effluents from off-shore oil rigs and careful monitoring in the vicinity of the rigs.
v) In the refinery, the emission of carbon dioxide, sulphur(IV) oxide, nitrogen oxides, solid particles etc. falls under the control of the National Alkali Inspectorate who require that "any discharge is avoided if possible". The company leaflet stated that "where complete prevention is not practicable, then the emission must be minimised".
One of the main harmful components of air-borne emissions from oil refineries is sulphur dioxide. Even in the late 1980s, in its main British refinery, this particular company recovered 100 tons per day of sulphur, though it did not state what percentage this represented. It did say that "it is not practicable to recover all the sulphur from refinery fuel, so the final emissions still contain some sulphur(IV) oxide. Tall chimneys are used to ensure adequate dispersion of flue gases, which are monitored by the National Survey of air pollution".
In a newspaper at the time ("The Planet") which accompanied a 1987 British Channel 4 television series, the following statements appeared:
a) "Today we have technical and economical means to combat all forms of air pollution. Only short-sighted greed and its political supporters stand in our way.
b) "One of the worst offenders is Britain. Favoured by westerly winds that blow across the North Sea, the UK dumps its sulphur(IV) oxide and nitrogen oxides on Scandinavia. It is the worst exporter of airborne pollution in Western Europe. It has opposed most of the international proposals to clean up Europe's foul air."
Airborne pollution causes acid rain which killed lakes and forests not only in Scandinavia, but also further south in Europe e.g. the Black Forest. (N.B. "The Planet" report is referring mainly to emissions from British power stations in Northern England.)
The situation has improved considerably since then.
Of course, if we wish to avoid personal responsibility, the above type of criticism is even more effective than criticism of companies. Governments are to blame. But in democratic countries it is we as individuals who elect governments, and such governments will not take too many actions that might lose them votes. (See paragraph on bin collections below.)
However, level of responsibility does increase with power. A typical Third World story illustrates the responsibility of governments and multinational companies. The poorest people in the World's poorest countries are often the people who suffer most in the face of financial constraints, whatever the source of those constraints. The story was once again reported in "The Planet".
In the 1950s the President of Brazil invited international companies to set up in Cubatao. The city was declared a military zone so that it would not be restricted by environmental laws.
By the time the article was written there was a democratic government in Brazil, and it had become clear that birth defects, respiratory diseases, cancers, and deaths in Cubatao were all connected with polluted air. More than half the children attended clinics to breathe supplies of medicated air without which they would have died.
Yet the pollution still continued. Most of the companies in Cubatao were multinationals producing goods for individuals like us in Europe and North America. Where did the responsibility lie?
v) Returning to the UK oil company, it used millions of gallons of water at its UK refinery. Before the water was returned to the sea, a variety of techniques were used to treat it: sour water stripping, gravity separation, and dual media filtration were used to meet the requirements of the Water Authorities or Harbour Boards (or company standards when these were more stringent). There was an ecological monitoring station next to the refinery, which showed that the company's system of water management was resulting in a continually improving situation.
vi) Some waste was treated in the refinery prior to discharge, while other waste, like toxic chemicals, were incinerated, or shipped to disposal sites licensed by the local authorities.
In several other industries, including the nuclear industry, the practice of dumping waste in the sea is used. Even back in the 1980s "The Planet" warned that the World's seas were threatened by huge increases in pollution, although using the sea as a dustbin has appeared reasonably successful in the past.
The report continued that scientists, policy makers and lawyers were divided on how dumping in the seas should be regulated. However, most of the countries who accepted the London Dumping Convention, agreed in 1983 and 1985 to adopt an indefinite moratorium on the dumping of radioactive waste at sea until those who wish to do it can prove that it is environmentally acceptable. The UK, France, Switzerland, South Africa, Canada and the USA opposed the moratorium and, of course, carried considerable economical and political weight.
The UK government has not always been a leader on environmental issues.
Victor Sebek, the marine biologist who made the "Planet" programme on dumping at sea, argued: "We cannot afford a relaxed bureaucratic attitude which restricts economic activities only after pollution has caused irreversible damage".
vii) The final section in the UK oil company's environmental leaflet said that all this activity to avoid or minimize impact on the environment is expensive. Like any other expense, this has to be included in the final price paid by consumers. Are we as individual consumers prepared to pay that price? If we are not prepared to meet the financial costs, that does not mean there is no price to pay, it just means we have less control over it.
And if this individual responsibility for pollution seems tenuous, are there more direct ways in which we encourage pollution, and what do we do with our own chemical pollutants? Given the choice between plastic disposable bottles, and glass bottles which we have to return for re-use, which would we buy? Have we ever left chemical litter, like plastic packaging, lying around? Have we ever thrown bleach down the sink? Do we buy bio-degradable cleaning products?
Clearly we at least control the extent to which our attitudes and actions either accept or reject the concensus attitude.
28.2.3. It is often tempting to think that pollution is only relevant to what we might see as the hard chemical industry.
i) We might, for example, see farming as a rather soft industry when it comes to polluting the environment. After all, farms are a part of the environment we are trying to protect from chemical pollution. However, agriculture uses chemicals in vast quantities, especially in richer countries. Examples are pesticides and fertilisers, which we might see as desirable chemicals. However, their manufacture may cause pollution, and so may their use.
Excessive use of pesticides has killed birds and animals which feed on the pests. Even the use of fertilisers, which are used to encourage plant growth, have harmful effects on both plant and animal life. When used excessively they tend to get washed out of the soil into streams and rivers. Here they encourage growth of bacteria. These in turn use up oxygen in the water and the lack of oxygen means that the streams and rivers are unable to support other plant and animal life. (See also Organic farming below.)
ii) Road traffic is another major source of pollution, though most of us would be reluctant to make many sacrifices when it comes to private motoring.
Even in an ideal world, the chemical reaction which produces the explosion that drives a vehicle (the combustion of a hydrocarbon fuel), results in the global warming gas, carbon dioxide.
But it's worse than
that. For complete combustion you would need ideal conditions:
• Sufficient oxygen
• Adequate mixing of the fuel
and oxygen
• The correct temperature
• Sufficient time for the
reaction
In practice the
explosion happens in a few milli seconds, determined by the time of the
engine cycle. Obviously, engine design is all about improving the
efficiency of this process, but combustion is not complete. The exhaust
fumes contain, alongside CO2 and water:
From incomplete combustion:
• Carbon monoxide (CO), a
poisonous colourless
and odourless gas.
• Hydrocarbons or volatile
organic compounds
(VOCs) and even carbon- containing soot. Sunlight breaks VOCs down to
form oxidants, which react with oxides of nitrogen to cause ground
level ozone, a major component of pollution.
From combustion of impurities:
• Oxides (NO and NO2)
of
nitrogen from the air. These contribute to smog and acid rain and also
cause irritation to human mucus membranes.
• Oxides of sulphur, which
also contribute to
smog and acid rain as well as causing irritation to human mucus
membranes. However, from 2006/7, the UK will start to move from the
current Ultra Low Sulphur Petrol and Ultra Low Sulphur Diesel fuels
(sulphur content not exceeding 50 parts per million or 0.005% by
weight) to sulphur-free petrol and diesel (sulphur content not
exceeding 10 parts per million or 0.001% by weight). This process will
start with diesel and super unleaded fuels, with all road fuels
switched by the start of 2009. No engine adjustments are required to
use sulphur-free petrol or diesel.
In order to reduce the three remaining types of pollutant, CO, VOCs and oxides of nitrogen, most modern cars are fitted with three-way catalytic converters. The converter uses two different types of catalyst, a reduction catalyst and an oxidization catalyst. Both types consist of a ceramic structure coated with a metal catalyst, usually platinum, rhodium and/or palladium. The converter is designed to expose exhaust fumes to the maximum surface area of the catalyst, while minimising the amount of catalyst required (since these are very expensive).
Three main structures are used for this purpose: ceramic honeycomb, metal plate and ceramic beads (now almost obsolete).
However, this still leaves CO2. Road transport accounts for 25% of total UK emissions of carbon dioxide (CO2), believed to be the major contributor to global warming and climate change. The EU has voluntary agreements with motor manufacturers that aim to reduce average CO2 emissions from new cars.
Colour-coded labels, similar to those used on washing machines and fridges, are now displayed in car showrooms showing how much CO2 new models emit per kilometre. And the level UK road tax on cars manufactured since 2003 depends on their efficiency. However, as traffic levels are predicted to increase, road transport will continue to be a significant contributor to greenhouse gas emissions, not least as developing countries demand to emulate the, so called, standard of living experienced in richer countries.
iii) Yet another source of pollution it would be hard to give up is plastics. As stated on the Graig Farm Organics website:
"Although plastics have been around since the 1930s, only around 5% of plastics are incinerated. As plastic is a very long-lived material, the planet is literally littered with 95% of all plastics which have been made. Obviously, in environmental terms, it is often better to find alternative materials to plastics. However, they remain extremely versatile and useful, and difficult to live without in today's world."
A simple solution might therefore appear to be to use biodegradable rather than non biodegradable plastics.
As a general rule:
• addition
polymers of alkenes and their derivatives are chemically inert and
therefore non biodegradable;
• condensation
polymers such as polyesters and polyamides can be broken down by
hydrolysis and are, therefore, biodegradable.
However, biodegradability might not be as tidy as it sounds (pun intended). As ever, there are other factors to consider such as the properties of the plastic needing to be appropriate to the function and, of course, economics.
Moreover, there's biodegradable and biodegradable. Some, so called, biodegradable plastics still take tens of years to break down.
Another part of the solution is reduction, recycling and re-use. According to Defra (The UK's government Department for Environment, Food and Rural Affairs):
"In 2001 there was 1,678,900 tonnes of plastic packaging in the waste stream, an increase from 1,600,000 in 2000. Research conducted in September 2000 indicated that UK consumers used eight billion plastic carrier bags per year.
The Government is taking action to support the reduction of plastic packaging in the waste stream, re-use of bags and recycling of plastics more generally.
UK manufacturers and retailers are increasingly introducing measures to reduce packaging and encourage re-use and recycling of plastic bags
However, at the end of the day, it is also up to us. There was evidence in 2007 local government elections that UK local local councils who had cut down refuse collections to encourage recycling, lost votes. Maybe we can't blame the government!
Recycling
is, of course, also relevant to other materials such as metals (see 19.1.4.viii
and 19.1.7.iii)
28.2.4. Many of the factors considered in the above debates may not seem to have much to do with chemistry. However, scientific arguments are used and abused by both sides in such debates. The abuse of scientific reasoning is particularly prevalent when economics and emotions enter the debate.
In late 1987, and in the wake of the Chernobyll nuclear power station disaster in Russia, decisions were still being made about an acceptable level of radioactivity in British lamb. On 5 November, a spokesman from the Ministry of Agriculture said on the BBC Radio 4 lunchtime news that an individual would need to eat 8 suspect lambs in one year before being subjected to the level of radiation experienced in one chest X-ray.
It was an interesting
comparison, but only because it was so misleading. There is a profound
difference between absorbing a 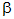 -
and
-
and 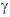 -emitting isotope
of caesium with a half-life of 30 years, and having a short dose of
X-rays passed through your body. Turning the light on would subject you
to electromagnetic radiation, but nobody would compare it with
-emitting isotope
of caesium with a half-life of 30 years, and having a short dose of
X-rays passed through your body. Turning the light on would subject you
to electromagnetic radiation, but nobody would compare it with 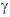 -
or even X-radiation (both of
which are electromagnetic), let alone with
-
or even X-radiation (both of
which are electromagnetic), let alone with 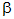 -radiation
which is completely different in nature.
-radiation
which is completely different in nature.
Supporters of the nuclear industry can be particularly creative in their use of comparisons. For example, by working out the number of people near a nuclear power station who can be proved to have died as a result of increased radioactivity, and by then working out the number of people in the same area killed in road accidents, they have been known to argue that living next to a nuclear power station is less risky than crossing the road.
Opponents of the nuclear industry are just as likely to abuse scientific arguments.
28.3. GOOD, BAD, OR
INDIFFERENT?
28.3.1. Food additives are currently receiving a great deal of criticism. We are told to avoid foods with a lot of E numbers in the list of ingredients, because these are synthetic additives and synthetic additives are harmful.
Food manufacturers have turned this to their advantage. Packaging proudly announces "No artificial flavourings, or preservatives, or colourings". Note that manufacturers rarely manage to exclude all three (FIG. 28.1.) "Natural products" are confidently assumed to be better for us than products which are not natural.

28.3.2. Does "natural" mean "harmless"? We do know that some synthetic additives are harmful. We do not know that all "natural" flavourings, colourings etc. are harmless. A major difference is that more research has been done on the harmful effects of chemical additives.
It is unscientific to assume that because a chemical has a natural origin it is automatically less harmful to us than one which does not have a natural origin. Perhaps it is less likely to be harmful, but even that has not been proved scientifically.
Sugar is a widely used natural flavouring, but it is certainly not harmless. In addition, sodium hydrogen L-glutamate which ironically started much of the interest in the harmful effects of additives, is obtained from sugar beet pulp and wheat gluten.
Thus sodium hydrogen L-glutamate ("monosodium glutamate", or "MSG") presumably counts as having a natural origin, but it has also been connected with "Chinese Restaurant Syndrome", the symptoms of which include, heart palpitations, headaches, dizziness, muscle tightening, nausea, weakness of the upper arms, pains in the neck, and symptoms similar to migraine in some people.
28.3.3. Moreover, how natural is natural? Often natural flavourings and colourings have been at least concentrated from their original source. There is a considerable difference between eating tomatoes and eating food which contains E160d, the natural food colouring Lycopene, which is extracted from tomatoes. Another example: does anybody actually eat Dactilopius coccus, the insect from which the chemical precursor of cochineal is extracted? Cochineal is listed as a natural food colouring. Many 'virtuous' savoury snacks claiming to contain only natural flavourings contain sea salt, an extaordinary cocktail of inorganic chemicals.
An oat breakfast cereal once available in the UK claimed to contain natural colouring. The natural colouring was caramel. Caramel (E150) does have a natural source, carbohydrates, but the most natural form is produced by the action of strong heat on the carbohydrate. The chemical reactions which result from the heating are extreme, and the brown colour is due to the presence of carbon. Its safety as a food additive is very much in question.
However, on the other side of the argument, we all cook natural foods without considering the products as synthetic. Cooking is a chemical process.
As a final thought, it is informative to remember that Deadly Nightshade is a natural plant, but eating it would be very unwise.
28.3.4. Does "synthetic" mean "harmful"? As for synthetic additives, even when they are known to have harmful side effects, these may be outweighed by the benefits. Which is worse, a dose of preservative, or a dose of chemical toxins produced by moulds or bacteria?
Moreover, some synthetic additives are even reckoned to be beneficial: E101, used as a yellow or orange-yellow food colouring, is usually prepared synthetically. It is better known as vitamin B2.
28.3.5. Final comment on food additives: There are many, so-called, natural additives which are not known to be harmful, but equally they are not known to be harmless. The same can be said of many synthetic additives.
Maurice Hanssen's book, "E For Additives" will provide some interesting insights.
28.3.6 Organic Farming: Similar flaky thinking is applied to organic farming. Many of the arguments which devotees use to support organic farming seem almost more religious than they are scientific. There seems to be a faith like belief that organic = natural = good and certainly = better than food produced using non organic farming methods.
As it happens there are still 4 Soil Association permitted chemicals available for use by organic farmers, such as copper sulphate to prevent potato blight. There are also 32 chemicals permitted in food production. Having said that, the equivalent figures for non organic farming are apparently 350 and 7,000 respectively.
There are, however, lots of questions which the organic sector still needs to answer, such as:
• Do we know all we need to
know about the
chemicals produced by the microorganisms and pests that might be
present in greater numbers in organic conditions?
• Do we know all we need to
know about the
effect on ecosystems of biological controls such as the introduction of
natural predators?
• If and when organically
grown produce tastes
better than food produced using conventional techniques, how much of
that is down to organic farming methods and how much is due to other
factors such as selection of tastier varieties? And how much of the
better taste is down to perception? Would the perceived differences
stand up to proper randomised tasting sessions? (See Fillion
and Arazi 2002, Nutrition & Food Science, Volume 32,
Number 4, pp. 153-157(5) or two 2007 articles from The UK Times: 2
April and 29
April)
• Is there scientific
evidence to back up the
claim that organic food is better for you? If there is, how much better?
• Could the population of the
world, especially
in poorer countries, be sustained using organic farming methods?
(See also 'other farming' above.)
28.3.6. Homeopathic medicines are also subject to some very unscientific criticism, mainly from scientists. Perhaps the most commonly misused phrase adopted to describe homeopathic medicines is, "There is no scientific basis for their action".
This usually means that, even if a particular homeopathic medicine were found to work, its action could not be described in terms of known mechanisms. This in turn could mean either that not enough is known about the homeopathic medicine, or that not enough is known about mechanisms, or both.
It rarely means that the homeopathic medicine has been shown not to work by a satisfactory randomised drug trial. However, it should also be added that when claims are made in support of homeopathic medicines, they too, are rarely (if ever) backed up by satisfactory randomised drug trials.
Nevertheless, it is at least possible, that the attribution of healing properties to some homeopathic medicines might result from observations by a large numbers of people over a long period of time. The observations may not have been recorded with scientific rigour, they may be coloured by subjectivity, but even so, this does seem to represent some kind of instinctive empirical science. If it does, it is more scientific than the dismissal of all homeopathic medicines by some scientists who cannot understand how they might work.
In a 2006 BBC2 television series in the UK, Professor Kathy Sykes said of alternative remedies that even if their main effect is bringing into play the Placebo Effect, maybe we are missing a trick by not exploiting this fact.
Moreover, if the effectiveness of alternative remedies depends, even in part, on belief in the remedy, how can that be measured using a standard randomised drug trial in which the recipient does not know whether they are receiving the remedy or not?
Perhaps we need new scientific methods. And if these show that some alternative remedies do work, it will then be the job of scientists to hypothesise about how they do work.
Please note, however, that there is no evidence that homeopathic remedies work other than by placebo effect.
28.3.7. Alcohol is an interesting chemical to discuss in a spiritual, moral, ethical, social, cultural (aqa syllabus section 17.1), economic or political context.
i) On the economic front, we saw in chapter 21 that alcohols can be produced chemically. The most commonly used method used is catalytic addition of water By recycling the ethane over the catalyst the yield can be increased to about 95%. About 5% is converted at each pass over the catalyst.
(NB other alcohols can be made using this method, but not all. For example, as predicted by applying the theory in section 20.14.2, propene gives propan-2-ol rather than propan-1-ol.)
Moreover, the majority of alcohol, even for industrial use, is manufactured using the biological process of fermentation. In the case of alcoholic drinks it is obvious why. For one thing, it would be difficult to imagine producing a fine wine or ale by mixing chemically produced alcohol with other ingredients.
Advantages of the synthetic process are that it is faster and it produces purer ethanol and, being a continuous flow process, is more efficient. The fermentation process, in contrast, is a batch process which requires containers to be emptied and ingredients replaced at the start of each reaction.
Moreover, it is very slow, and even the most resistant strains of yeast are killed when the alcohol concentration reaches 15 - 18%. Higher concentrations of alcohol (whether for 'spirits' to drink or for industrial purposes) must be obtained by distillation. The limit is 96% alcohol [an 'azeotropic' mixture (see section A3.3.4.iv)].
However, fermentation has two advantages:
First it is carried out at low temperatures and at atmospheric pressure compared with high temperature, high pressure and high energy input for the synthetic process.
Secondly, it uses renewable resources from plant materials whereas the synthetic process uses finite resources from crude oil.
Such plant based renewable resources are very much in favour in 2007. Alcohol from plant sources is seen as part of the answer to the great energy debate, not just because it is seen as a renewable resource, but also because alcohol is a cleaner fuel than oil based hydrocarbons.
However, questions need to be asked about the real sustainability of industrial scale bio-fuel manufacture. Can the world afford to turn over agricultural land currently used for food production? If not, where is land on this scale going to come from and what impact will that have? It is important to remember the devastating effects when vast tracts of rain forest were converted into agricultural land for food production.
Update 7 July 2008:
A panel of UK government experts, chaired by Professor Ed Gallagher,
head of the Renewable Fuels Agency, has looked at the impact of energy
policy on land use.
Its report calls for biofuels to be
introduced more slowly than planned until controls are in place to
prevent higher food prices and land being switched from forests or
agriculture.
It predicts that current policies could see grain prices in the EU rise
by 15%, sugar by 7% and oil seed by 50%.
The
review estimates that an extra 10.7 million people in India could find
themselves in poverty, while countries such as Kenya, Malawi and
Bangladesh could see hundreds of thousands affected. Follow this link
to the BBC News website for further information.
Even so, the balance is that most alcohol is produced by fermentation.
The starting point is usually starch from a plant source such as barley, wheat, potatoes or what the US calls corn and the UK calls maize, though there are schemes using sugar cane for bio-fuel production, e.g. in Brazil.
Starch (see section 25.3.5.ii) is broken down by using malt. Malt is germinated barley and it contains the enzyme maltase. As the name suggests, maltase breaks the starch down into a simpler carbohydrate, maltose (see section 25.3.4.).
Yeast is then added to the mixture and enzymes in the yeast convert disaccharide sugars such as maltose and sucrose into even simpler sugars. Other enzymes then convert the monosaccharides into alcohol.
The chemical reactions in fermentation are complex, but they can be summarised:
C12H22O11.... +.... H2O.... →
.... 2C6H12O6
C6H12O6.... →
.... 2CH3CH2OH.... +.... CO2
(NB Other yeast enzymes also break sugars down into carbon dioxide and water. The variety of yeast and the conditions determine the ratio. Baker's yeast produces more carbon dioxide and it is these bubbles of carbon dioxide which cause dough to rise. There are many different varieties of yeast used in the drinks industry all with different characteristics, including differing alcohol tolerances. Note also, that bacterial infections and too much air can result in the alcohol being oxidised to carbonyls and even as far as ethanoic acid, vinegar. This needs to be prevented. However, some of the flavours in fruitier ales may well be due to oxidation products such as aldehydes.)
ii) Social
impact: The social effects of alcohol and the effects on
mental health are well documented. Binge drinking is seen by
some as a scourge of the
early 21st century.
To select but a few statistics, a report published by the Portman
Group,
a group set up by the drinks industry in response to public and
government criticism:
• A
quarter of all adults claim to have been a victim of alcohol-related
violence themselves, in a pub (14%), on the street (4%), or in their
homes (7%).
• Street
drinking was felt to be a problem by seven out of ten people surveyed
with the majority (57%) seeing teenagers as the worst offenders
• More
than half those surveyed thought that alcohol-related crime was still
increasing, both on the street (61%) and in the pub(52%)
According to Alcohol
Concern:
• Alcohol
use is associated with 70 % of all stabbings and beatings;
And Home
Office figures reveal that:
• Victims
of violence judged offenders to be under the influence of alcohol in
40% of incidents
• 53%
for stranger violence - reflecting that it often happens near a pub or
club
• the
percentage was lowest for mugging (17%), possibly reflecting the
premeditated nature of the crime.
We also know that
drink driving is responsible for death and injury on the roads. The
most recent figures on the Road
Safety Week 2007 website , are *not encouraging. Provisional estimates for 2002 from the
Department for Transport show that between 1993 and 2002:
• The
number of crashes caused by drivers over the legal limit increased by
39%, from 9,480 to 13,150
• The
total number of casualties caused by drink-drivers increased by 34%,
from 14,980 to 20,140
The breakdown of
casualties by severity over that decade is:
• The
number of people killed in drink-drive crashes increased by 3.7%, from
540 to 560
• The
number of people seriously injured in drink-drive crashes increased by
6%, from 2,660 to 2,820
• The
number of people slightly injured in drink-drive crashes increased by
42%, from 11,780 to 16,750
But where does the
solution lie? The Portman
Group report referred to above also shows that:
• Whilst
80% would support a ban on drinking in some public areas, almost half
say they have no confidence that the police would be able to enforce
the ban effectively.
Again, the answers rest with individuals as well as with government, law enforcement agencies and the drinks and entertainment industries. However, these agencies do have a role to play and on 28 May 2007 the BBC reported: 'Alcoholic drinks will carry new health warning labels by the end of 2008 under a voluntary agreement between ministers and the drinks industry.' The BBC web page also carried online video footage showing the response from shoppers.
*NB
Some police forces in the UK reported some improvements over the
Christmas 2006 period. For example, during a nationwide campaign to
combat drink driving Kent saw arrest figures fall during December 2006
compared to the same period in 2005.
Hopefully, this book will have helped you to become a more thinking chemist. Much difficulty with chemistry is caused by lazy thinking. Perhaps this last chapter may also have lead you to believe that lazy thinking affects more than just exam performance.
Ben Goldacre's book, Bad Science, and his eponymous Guardian newspaper column may also help you to keep science in general in perspective.
28.6. QUESTIONS
1) What actions by you, your family or your friends pollute the environment? Describe how any of those actions could be avoided.
2) What criteria should be used to decide when the financial cost of preventing industrial pollution outweighs the benefits?
3) Why do many people consider radioactive pollution more threatening than chemical pollution? Is it more threatening? Explain.
4) Using section 28.2.2.iv. as your only source of information, where does the responsibility lie for the situation in Cubatao? Explain your reasoning, and say what other information might lead you to modify your opinion.
5) What do you think the procedures described in section 28.2.2.v. (i.e sour water stripping etc.) are? What is your opinion of the terminology used to describe them?
6) Why is it unreasonable to compare risks from radiation or chemical pollution with, say, the risk attached to crossing the road? What useful risk comparisons do you think could be made?
7) Why, do you think, would many people feel happier about salt and vinegar on their chips, than they would about 6-0-Palmitoyl-L-ascorbic acid (E304) in their sausages? Salt has been associated with heart disease whereas there are no known harmful effects of E304, an anti-oxidant and colour preservative.
8) A scientist did not know if a certain plant extract fulfilled the claims made for its healing properties. Nor could she explain its action in terms of her own understanding of pharmacological mechanisms. However, the homeopathic medicine had a long history of use, and the scientist decided that it would be a fruitful subject for research. What types of research do you think she might have carried out? If the first stage of her research had produced statistical evidence that the medicine worked, what effect would that have had on her theories of drug action, and what further research would have been useful?
Unless otherwise stated, all materials in this web version of chapter 28 are © 2007 Adrian Faiers MA (Oxon) MCIPR

What 's the connection between a dozen eggs and
a garden mole?

Answer: Not a lot, really, but see Chapter 1